Nanoemulgel for Transdermal Delivery of Cyclobenzaprine Hydrochloride: Design, Characterization and In-Vitro Studies-Juniper Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
Recently utilizing nanoemulsion as a vehicle for
deliver the drug through transdermal drug delivery has been increased.
The purpose of the present study was to develop a nanoemulsion
formulation of cyclobenzaprine hydrochloride and characterize for
transdermal drug delivery. Aqueous titration method was adopted for
various oil-in-water nanoemulsion preparations. The prepared
nanoemulsions were characterized for its particle size, polydispersity
index, zeta potential, TEM, pH, viscosity, drug content, spread ability
and its in-vitro release studies. Drug excipient compatibility
study results reveals that the excipient used in the nanoemulsion is
having compatibility. Based on its in- vitro release studies
formulation F14 showed higher release compared to other formulations.
Formulation F14 was converted into Nanoemul gel and characterized. The in-vitro
release profile of the optimized formulation was compared with the
nanoemul gel. In conclusion, the results of the present investigation
suggested that the developed nanoemul gel formulation of cyclobenzaprine
hydrochloride can be used as a vehicle for enhancement of
bioavailability through transdermal drug delivery.
Keywords: Diabetes mellitus; Kaempferitrin; Hyperglycemia; PlantsCyclobenzaprine hydrochloride; Nanoemulsion; Nanoemul gel; Release studies.
Introduction
An ideal drug delivery system is that fulfills the
objective of spatial placement and temporal delivery resulting maximized
therapeutic effect and least toxicity. The pharmaceutical scientists
are using a wide range of methods to improve the bioavailability of
poorly water-soluble drugs coming out of high throughput screening in
drug discovery process. With the progress in time and growth of science
and technology, the dosage forms have evolved from simple mixtures and
pills into highly sophisticated technology intensive systems, which are
known as novel drug delivery systems (NDDS). Different approaches have
materialized into various forms of NDDS such as microemulsions, multiple
emulsions, liposomes, niosomes, micospheres, pharmacosomes, virosomes,
dendrimers, etc., Most often the problems associated with these delivery
systems are their stability and predictability in biological systems
which reduce their clinical potential, although each one is associated
with its own strong points [1,2].
Nowadays Nanotechnology is a rapidly expanding field.
Various nano-pharmaceuticals currently being used in the process of
development are nanoemulsions, nanosuspensions, nanospheres, nanotubes,
nanoshells, nanocapsules, lipid nanoparticles and dendrimers [3].
In recent years, one of the most popular approaches is the
incorporation of the active lipophilic component into the inert lipid
vehicles [4].
Among these drug delivery systems Nanoemulsions has emerged as a boon
of nanotechnology in the form of a novel drug delivery system (NDDS).
Nanoemulsions are a group of dispersed particles used for
pharmaceuticals, biomedical aids, vehicles and also the most valuable
options offered to improve the oral bioavailability of poorly
water-soluble drugs [5].
Nanoemulsion is considered to be a thermodynamically or kinetically
stable liquid dispersion and transparent that shows great promise in the
future pharmaceuticals. The most used type of nanoemulsion in
pharmaceutical is the system in which the oil (internal) phase is
dispersed in an aqueous (external) phase [6].
In Nanoemulsion the drug is loaded into the inner
phase of these systems and delivered by lymphatic route, bypassing the
enzymes in the gastrointestinal tract (GIT) and reducing the pre
systemic clearance and hepatic first pass metabolism. Nanoemulsions have
a higher solubilization capacity, better thermodynamic stability, long
self-life, rapid onset of action, the nanosized droplets leading to
enormous interfacial areas of the drugs associated with nanoemulsions
would influence solubilization behavior, transport properties as well as
absorption across the mucosa are an important promising factor to
achieve sustained and optimum targeted drug delivery [7,8].
Nanoemulsions have been reported to improve the plasma concentration
profiles and reduced inter subject bioavailability of drugs more
reproducible [9-11].
Cyclobenzaprine hydrochloride is a skeletal muscle
relaxant used in the treatment of skeletal muscle relaxant. It is
extensively metabolized and excreted primarily as glucronides via kidney
and has slow elimination half life of 18h and an oral bioavailability
of 33% to 55%. The daily dose ranges from 5 mg to 10mg. It is
commercially available as oral tablets. In the present study, to
overcome the obstacles in oral drug delivery and to enhance the
bioavailability of the cyclobenzaprine, we have developed a novel O/W
type nanoemulsion based gel formulation for the transdermal delivery of
cyclobenzaprine hydrochloride without the use of additional permeation
enhancers for better applicability, to enhance the local therapeutic
potential through the skin.
Materials and Methods
Drugs and chemicals
Cyclobenzaprine hydrochloride was obtained as gift
samples from Hetero Pharmaceuticals Pvt Ltd., Hyderabad, India; tween
80, span 80, polyethylene glycol, propylene glycol, carbopol 940, HPMC
K15 and isopropyl myristate were procured from Loba Chemie Pvt., Ltd.,
Mumbai, India; methanol, ethanol, potassium dihydrogen phosphate, sodium
hydroxide, hydrochloric acid, triethanolamine and sodium chloride were
purchased from SD fine chemicals, Mumbai, India; Dialysis membrane was
procured from Sigma Aldrich, USA.
Screening of components
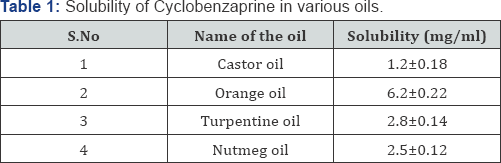
The solubility of cyclobenzaprine in various oils
(castor oil, orange oil, turpentine oil and nutmeg oil), surfactant
(tween 80) and co-surfactants (span 80, polyethylene glycol, propylene
glycol and ethanol) were determined by dissolving excess amount of
cyclobenzaprine in 2ml of each of the selected oils, surfactants and co
surfactants in 5ml capacity stoppered vials separately and mixed for 10
minutes using a vortex mixer. Combination of oils was also taken for
determination of solubility. The mixture containing vials were then kept
at 37 °C in an isothermal shaker (Nirmal International, Delhi, India)
for 24 hours to attain equilibrium. The equilibrated samples were
centrifuged at 3000rpm for 15 minutes. The supernatant was taken and
filtered through a 0.45μ membrane filter and the concentration of
cyclobenzaprine was determined by UV spectrophotometer at 290nm. The
solubility study is shown in Table 1.
Pseudo ternary phase diagram study
On the basis of the solubility studies orange oil was
selected as oil phase. Tween 80 and ethanol were selected as surfactant
and co surfactant respectively. Distilled water was used as an aqueous
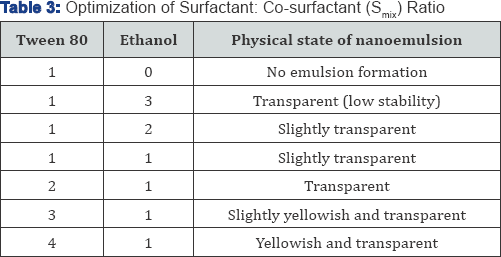
phase. Surfactant and co surfactant (Smix) were mixed in different
ratios (1:3; 1:2; 1:1; 2:1; 3:1 and 4:1) were used to optimize and
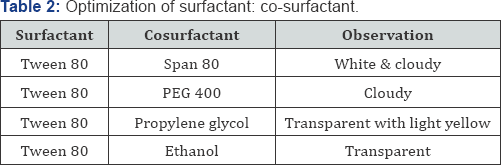
determine the optimum ratio. Optimization of surfactant and co
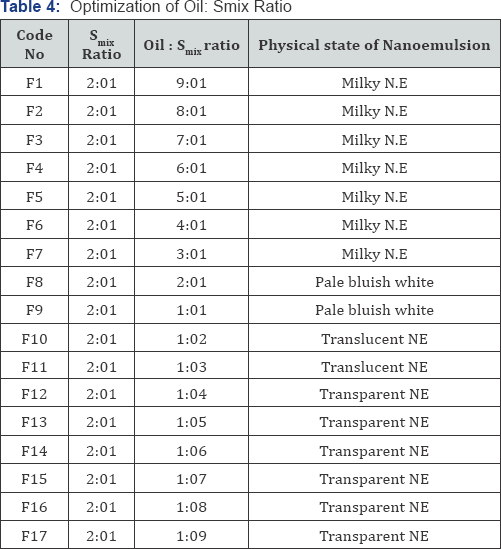
surfactant is shown in Table 2. For each phase diagram, oil and specific
Smix ratio was mixed well in different weight ratios from 1:9 to 9:1 in
different glass vials. Seventeen different combinations of oil and Smix
, 1:9, 1:8, 1:7, 1:6, 1:5, 1:4, 1:3, 1:2, 1:1, 2:1, 3;1, 4:1, 5:1, 6:1,
7:1, 8:1 and 9:1 were made so that maximum ratios were covered for the
study to delineate the boundaries of phases precisely formed in the
phase diagrams. Pseudo ternary phase diagrams of oil, Smix and aqueous
phase were developed using aqueous titration method under magnetic
stirring [12].
Slow titration was done after each addition of aqueous phase to each
ratio and observed visually for its transparency in terms of clarity.
Optimization of surfactant: co surfactant and oil: Smix ratio is shown in (Table 2-4).
Drug-excipients compatibility studies
Excipients are integral components of all dosage
form. Compatibility between the drug and excipients are determined by
DSC, FT-IR, TLC and UV techniques. Fourier Transform Infrared
Spectroscopy (FTIR) and UV spectrophotometry studies were used for the
determination of physicochemical compatibility and interactions between
the drug and excipients. The earlier reports on drug-excipient
interactions recommended that 1:1 ratio of drug and excipient maximizes
the possibility of interaction and helps in easier detection of
incompatibilities [13].
Therefore, in the present study 1:1 ratio was used for the preparation
of physical mixtures and analyzed for compatibility studies.
Preparation of cyclobenzaprine nanoemulsion
Homogeneous organic phase was prepared by dissolving drug in the oil phase. Then the selected Smix
was added to the oil phase and stirred in the magnetic stirrer for 5
minutes. The homogenous organic phase was slowly injected into the
aqueous phase under magnetic stirring (4000rpm); the o/w emulsion was
instantaneously formed by diffusion of the organic solvent in the
external aqueous phase leads to formation of nanodroplets and to attain
the equilibrium. Magnetic stirring was continued for 8 hours at room
temperature for the complete evaporation of the water miscible solvent.
Preparation of cyclobenzaprine nanoemulgel
Cyclobenzaprine nanoemulgel was prepared by
dispersing 1g of the Carbopol-934 in sufficient quantity of distilled
water, after complete dispersion of the polymer it was kept in dark for
24 hours for swelling. Then the cyclobenzaprine loaded nanoemulsion was
slowly added to the viscous solution of Carbopol-934 under magnetic
stirring. Other ingredients isopropyl alcohol and PEG-400 were added and
mixed well; then the pH was adjusted to neutral with triethanolamine.
The composition of cyclobenzaprine nanoemulgel is given inTable 5.
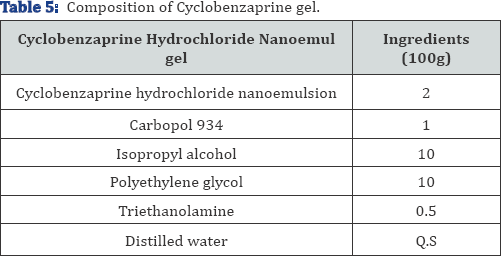
Characterization of cyclobenzaprine nanoemulgel
Globule size distribution & polydispersity index:
The mean globule size of the nanoemulsion was determined by Photon
Correlation Spectroscopy (PCS) using a Zeta sizer 3000 (Malvern
Instruments, UK). This analytical results reveals the mean diameter of
the particle at 25 °C, and at an angle of 90 degree (n=10). The PCS
analysis yields a mean diameter (z-average) as a light intensity-weighed
size of bulk population and the polydispersity index as a measurement
for the width of a globule size distribution.
Zeta potential: The electrophoretic mobility
was obtained by a Laser Doppler Anemometer connected with the Malvern
zeta sizer instrument. A suitable amount of sample (50-100μL) was
diluted with 5mL of water (0.45μm) and injected in the electrophoretic
cell of the instrument where a potential of ±150mV was set. The zeta
potential values were calculated by the instrument software using
Smoluchosky equation.
Morphology by transmission electron microscopic (TEM)
TEM helps to visualize the inherent matrix of
individual globule and its shape. A drop of the suitably diluted sample
was placed on to a holey carbon coated copper grid and left for 10
minutes. Then grid was kept inverted and a drop of phosphotungstic acid
(PTA) was applied to the grid for 10s. Excess of PTA was removed by
absorbing on a filter paper and the grid was analyzed using the
TECNAI-10 (PHILIPS) operated at 70-80kV at 17500 x magnification.
pH: The pH of the formulation was measured in a pH meter (Sartorius, Germany).
Viscosity: The viscosity of the nanoemulgel
was determined by a viscometer (Brookfield DV-E viscometer,) which was
rotated for 10min at 100 maximum rotations per minute with spindle 61
(Shivhare, 2009).
Drug content: The amount of drug present in
the cyclobenzaprine nanoemulgel was determined by dissolving 5g of
nanoemulgel in 25ml of phosphate buffer solution at pH 7.4 by
sonication. After sonication the solution was filtered, the filtrate was
diluted suitably and the absorbance was measured at 273nm in UV-Visible
spectrophotometer (Shimadzu, Japan, Model: 1700), finally the
percentage of drug content was calculated.
Spreadability: Spreadability test was
performed by measuring the spreading diameter of the nanoemulgel between
two glass slides. Weighed about 0.5g of sample and placed at the centre
(within a circle) of the glass plate, over which a second glass plate
was placed and pressed between the two slides and measured the
spreadability of the gel in cm after 5 minutes.
In-Vitro release
In-vitro release studies were carried out by
using dialysis membrane bag method. The dialysis membrane was
conditioned by soaking in phosphate buffer 7.4 for 8 hours.
Cyclobenzaprine nanoemulgel of about 3mL was taken in the dialysis
membrane and immersed in 200mL of phosphate buffer solution (pH 7.4). A
sample of 5mL was withdrawn from the dissolution setup at regular
intervals for 8 hours and an equal volume of phosphate buffer (pH 7.4)
was replaced to maintain a sink condition. Samples were analyzed by
using UV spectrophotometer at 290nm and the amount of drug release was
calculated and compared with the marketed oral dosage form.
Result and Discussion
Formulation in the form of Nanoemulsion has higher
solubilization capacity, better thermodynamic stability, long self-life,
rapid onset of action and large interfacial areas for the drug action
leads to provide optimum targeted drug delivery and reduced inter
subject bioavailability variation. The drug cyclobenzaprine is a
skeletal muscle relaxant, its pharmacological property suggest that it
can be a very good potential drug candidate for the transdermal drug
delivery. Our present study is to formulate nanoemulgel for transdermal
drug delivery of cyclobenzaprine. The materials used in the formulation
are chosen based on its acceptability, nonirritant and nonsensitizing to
the skin. Oils, surfactants and co-surfactants are having different
physico-chemical properties, but in combination with each other modify
the characteristics of the resultant self-emulsifying drug delivery
systems. The selected oil phase has important role that the drug should
be maintain in the solubilized form. Non-ionic surfactants with relative
high HLB value are widely recommended to form a stable nanoemulsion
formulation due to its high hydrophilicity which assists the immediate
formation of o/w droplets and rapid spreading of the formulation in
aqueous media. Surfactant alone some time may not able to give clear and
stable nanoemulsion. If the formulation is slight cloudy it may show
phase separation after few days, it can be avoided by inclusion of
hydrophilic co-surfactant. This co surfactant produces clear and aqueous
stable nanoemulsion of interest. Ethanol was selected as co-surfactant,
which has HLB value of 4.2. Screening of the components for the
solubility of the drug in the oil, surfactant and co-surfactant are
having important role. Solubility study was performed in different oils,
surfactant and co-surfactant. The solubility of the cyclobenzaprine was
found to be highest in orange oil when compared to the other oils. The
drug was completely soluble in tween 80 and highest solubility of the
drug was observed in ethanol. Hence, orange oil, tween 80 and ethanol
were selected as oil, surfactant and co surfactant respectively for the
phase study.
Constructing phase diagram is time consuming and care
is required to be taken to ensure the observations are not made on meta
stable systems. Concentration of compounds for the preparation of
nanoemulsion can be easily determined from phase diagram. Safety is a
major determining factor in choosing a surfactant, as a large amount of
surfactants may cause skin irritation. Hence from the phase diagram
study results, the oil: S mixratio of 1:5, 1:6 and 1:7 were
chosen for the further studies. The required quantity of the drug was
incorporated in nanoemulsion and observed visually for its transparency
and flow ability. Transparency of the nanoemulsion is shown in Figure 1.

FT-IR and UV studies were performed to investigate
chemical interactions between drug and the excipients. The FT-IR of
cyclobenzaprine shows intense bands at 3000, 2980, 1500, 3100 and 860cm-1
corresponding to the functional groups of alkenyl stretching, alkyl
stretching, NH bending vibration of the tertiary amino group, aromatic
CH stretching and bending vibrations respectively; these characteristic
bands were not appeared in the formulation this might be due to the
complete entrapment of the drug in the formulation excipients. No new
bands or shift in characteristic peaks were appeared. In UV technique,
the UV spectrum of drug is super impossible with the spectrum obtained
with drug excipients mixtures and there is no change in the λmax
of 290nm between the drug and drug excipients mixtures. FT- IR and UV
results revealed that there is no chemical interaction between the drug
and the excipients used in the formulation and found to be compatible
with each other (Data not shown).
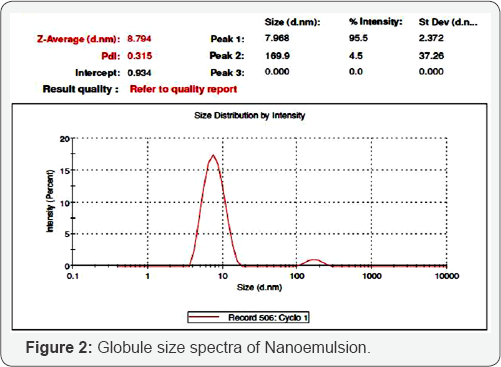
The polydispersity (PDI) of optimized formulations
F12, F13 and F14 were measured and the value was found to be 0.315,
0.177 and 0.175 respectively; which indicates the broad distribution of
globules and its homogencity. Globule size in the formulation either
increases or decreases which can affect the drug release from the
formulation and consequently its bioavailability. For emulsion based
products, the globule size of droplets of the internal phase have an
impact on the stability of emulsion itself. The mean globule size was
found to be 38.79nm, 24.55nm and 8.79nm for formulation F12, F13 and F14
respectively. The globule size distribution for the formulation F14 is
shown in Figure 2.
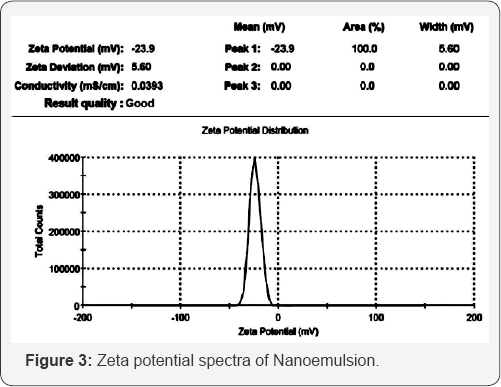
The zetapotential was determined by using Malvern
zetasizer, it was found to be -23.9mV, -20.4mV and -18.45mV respectively
for formulation F12, F13 and F14 respectively. Nanoemulsion formulation
consists of non-ionic surfactants which show relatively neutral charge,
it means it will not affected by body membrane charge during
absorption. The formulation shows an accepted value for good stability,
the zeta potential distribution is shown in Figure 3.
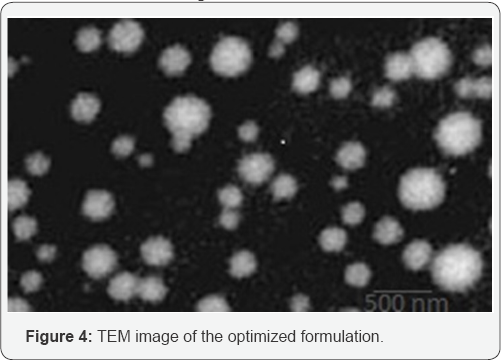
TEM for the prepared formulations was examined using
TECNAI-10 (PHILIPS) operated at 70-80kV at 17500 x magnification. The
TEM results showed that the globules were of nanometer in size range
with uniform, spherical and smooth surface. The TEM image of the
formulation F14 is shown in Figure 4. The pH values of the prepared
formulation were found to be between 6.13 and 6.55 nearing neutral. The
result reveals that the pH of the prepared Nanoemulsions is within the
acceptable limits for topical application.
The viscosity of the formulation was found to be
between 38.28cps and 45.25cps. The viscosity was decreased when there is
an increase in concentration of oil. The observed results are matching
with the earlier reports [12].
Drug content of all formulations was in the range between 97.54 and
101.25% w/w. Spreadability is an important parameter for the gel it
should comes out from the tube. The spreadability of nanoemulgel
formulation was determined; which exhibit the good spreadability when it
applied on the inflamed skin with maximum slip and drag. The
spreadability of the formulation was found to be 4.6±0.18gcmS-1.
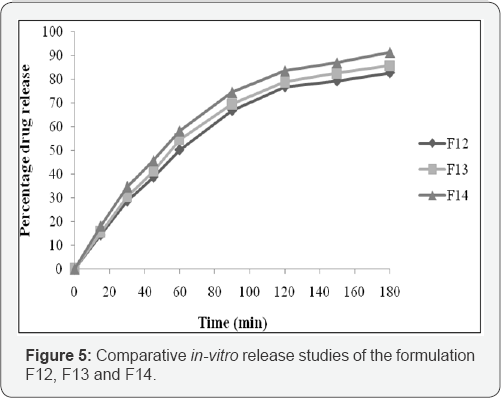
The in-vitro release profile of
cyclobenzaprine nanoemulsion is given in Figure 5. A burst release
followed by a steady release has been observed and the drug release
process ranked in the order of F14>F13>F12. The amount of drug
released after 180 min were found to be 91.22%, 85.66 and 82.63%
respectively for F14, F13 and F12. Thus significant (P<0.5, t-test)
higher drug release was observed for formulation F14 compared to
others. The optimized formulation F14 followed the higuchi release
kinetics that is based on diffusion controlled. According to the "n"
value 0.158 obtained from korsemeyer peppas model, F14 followed
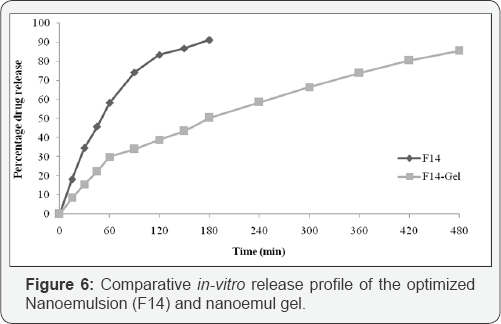
non-fickian case II diffusion mechanism. The in- vitro release profile of cyclobenzaprine from its nanoemulgel formulation is compared with its nanoemulsion. The comparative in-vitro release is shown in Figure 6.
Conclusion
Cyclobenzaprine is drug used as skeletal muscle
relaxant. Recently formulation in the form of nanoemulsion has attention
in the drug delivery system due to its nanosize droplets with good
stability. A suitable combination of the oil, surfactant and
co-surfactant are the major consideration in the formulation of
Nanoemulsion as transdermal drug delivery system. In this work,
cyclobenzaprine nanoemulsion has been developed. The optimized
nanoemulsion formulation, which exhibited maximum drug release, was
formulated as novel nanoemulgel of cyclobenzaprine with suitable
viscosity for topical administration. The in-vitro results
revealed that the developed nanoemulsion based gel containing
cyclobenzaprine hydrochloride has great potential to provide better
therapeutic effect locally through topical application.


Comments
Post a Comment