Novel Approaches for Treatment of Breast Cancer Tumors -Juniper Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
In the last few years, various new drugs have been
introduced on the international markets to treat breast cancer. These
drugs are administered either orally or via an injection. In this
article, we provide an insight into the current pipeline of drugs
introduced during the last seven years. These drugs minimize toxicity
and improve efficiency. They offer greater benefits to patients and have
reduced side effects.
Introduction
Breast cancer is the most commonly diagnosed cancer among females worldwide, with an estimated 14.1 million reported cases [1].
Cancer cells form a tumor when they have lost the ability to stop
reproduction and enter the death phase at the proper time. A solid tumor
comprises two parts:
a. Tumor parenchyma.
b. The stroma. The stroma contains the blood vessels
and other supporting cells. As the tumor grows, the pre-existing blood
vessels experience increased pressure which limits blood flow.
The idea of targeted killing of a cancer tumor
originated in 1906 when Ehrlich introduced the concept of drug targeting
of cancer cells by tissue-specific carriers that can deliver toxic
agents to neoplastic tissue [2].
The advancements in nanotechnology have revolutionized drug delivery in
oncology by introducing advanced therapeutic systems for cancer
treatment [3].
There are currently three different signal transduction pathways that
are targeted for the purposed of adjuvant breast cancer treatment by the
use of hormone-blocking agents, chemotherapy and monoclonal antibodies.
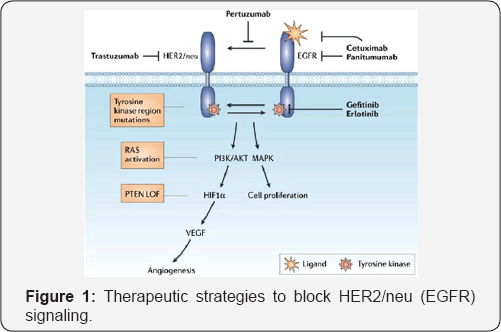
Targeting of EGFR (HER2)/neu signaling pathway
The human epidermal growth factor receptor (EGFR or HER2) is a tyrosine kinase receptor (Figure 1). These proteins serve as cell surface receptors in healthy cells and play important roles in signal transduction pathways [4]. Currently, Trastuzumab (Herceptin®) [5], a monoclonal antibody inhibitor and Lapatinib (Tyverb/Tykerb®) [6],
a dual EGFR/HER2 kinase inhibitor, are used for the treatment of HER2-
positive cancers. Small molecule tyrosine kinase inhibitors have also
provided attractive therapeutic agents, as they are able to block cell
signaling associated with many of the proposed mechanisms for HER2
resistance.
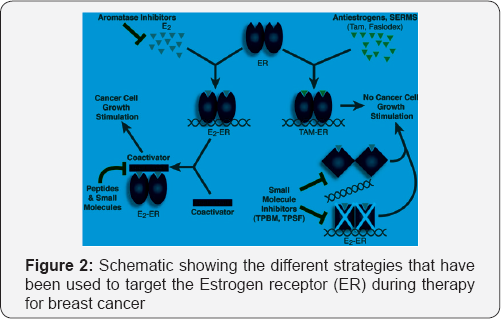
Targeting of estrogen receptor (ER) signaling pathway
The majority of breast cancer tumors over-express the
estrogen receptor (ER)α which regulates transcription and drives
estrogen-stimulated proliferation of ER+ tumor cells (Figure 2) [7].
ER + patients usually receive adjuvant antiestrogen therapy that relies
upon on ER modification, down- regulation, or estrogen depletion [8].
Tumors often develop resistance to anti-estrogen therapy through
stimulation of ER itself or of downstream mediators of ER-driven
transcription,as well as activation of alternative proliferation
pathways, in particular those driven by HER2/EGFR.
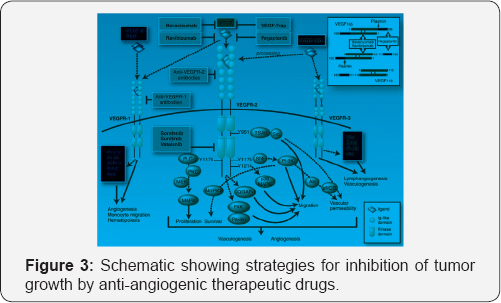
Targeting of vascular endothelial growth factor receptor-2 (VEGFR2) signaling pathway
Vascular endothelial growth factors (VEGF) and their receptors play an important role in blood vessel formation (Figure 3) [9,10]. Mice lacking various VEGF ligands or receptors show defects in vascular formation and maturation [3].
Members of the VEGF family are also involved in other biological
processes, such aslymphangiogenesis, vascular permeability, and
hematopoiesis. VEGF is released by tumor cells and induces tumor neo
vascularization.
Conclusion
Over the past seven years, significant improvements
have been made in breast cancer treatment. The development of the field
of molecular biology has made the treatment of different types of breast
cancers more efficient. Drugs are now being designed to target
molecules of signaling pathways that are important for cancer cell
survival and proliferation. Estrogen receptor and HER2 signaling
pathways have emerged as the most important targets for these drugs.
Anti-HER2 targeted therapies have improved survival rates by 15%-23% in
patients suffering from HER2 over-expressing breast cancers.
Acknowledgement
The authors wish to acknowledge the approval and
support of this work by the grant no. 435-014-2 from the Deanship of
Scientific Research at Northern Border University, Arar, KSA.
For more articles in Novel Approaches in Drug
Designing & Development
please click on: https://juniperpublishers.com/napdd/index.php


Comments
Post a Comment