Synthetic N-(Alkyl/Aralkyl)-N-(2,3-Dihydro-1,4-Benzodioxin-6-Yl)-4-Methylbenzenesulfonamides as Acetyl cholinesterase Inhibitors-Open Access Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
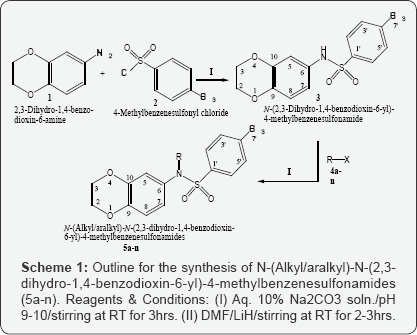
The current research effort involved the reaction of
2,3-dihydro-1,4-benzodioxin-6-amine (1) with 4-methylbenzenesulfonyl
chloride (2) in the presence of 10% Na2CO3 under
dynamic pH control to obtain
N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-methylbenzenesulfonamide (3)
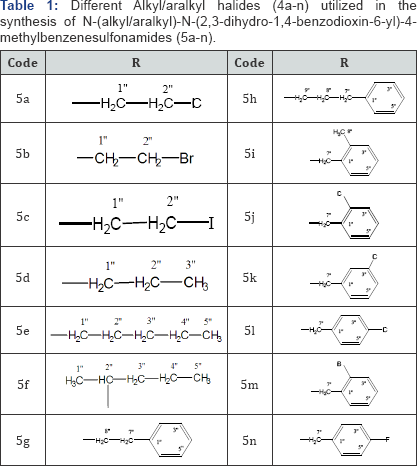
which was further coupled with a series of alkyl/aralkyl halides (4a-n)
to attain N-alkyl/aralkyl-N-(2,3-dihydro-1,4-benzodioxin-6-yl)-
4-methylbenzenesulfonamides (5a-n), using polar aprotic solvent; DMF and
catalytic amount of lithium hydride as base. The structural
characterization of these newly synthesized compounds was done by IR,
1H-NMR and EIMS spectral data. All these compounds were assessed for
their acetyl cholinesterase inhibitory potentials and were found to be
moderate inhibitors of this enzyme. Two molecules, 5f and 5n displayed
excellent to effective inhibitory potential respectively while the other
showed moderate inhibitory potential against acetyl cholinesterase
enzyme.
Keywords: 2,3-Dihydro-1; 4-Benzodioxin-6-amine; Sulfonamides; Spectral analysis; Acetyl cholinesterase Introduction
The basic sulfonamide group SO2NH is found
in numerous biological active compounds including antiviral,
anticancer, anti-thyroid, antimicrobial, anti-inflammatory and
antibiotics drugs along with inhibitors of carbonic anhydrase [1]. Because of their less cost, less toxicity and astonishing activity, these are extensively used as antibacterial agents [2-6].
Furthermore, sulfonamides are also employed as, anti-leprotic,
diuretics, antitumor agents, tuberculostatics and oral hypoglycemic
drugs [7-8]. Aliphatic sulfonamide derivatives act as antifungal agents [9]. Sulfonamide based antibiotics are utilized as veterinary medicines to treat infections in livestock herds [10]. Compounds bearing benzodioxane ring system exhibits different biological activities such as anti-oxidant [11], anti-hepatotoxic and anti-inflammatory [12,13]. Aryl sulfonamides having benzodioxane moiety have been recognized as inhibitors of ExoU [14].
Because of their non-interaction to defence mechanism of host and broad
spectrum activity some effective derivatives of sulfonamides are widely
used to treat gastrointestinal and urinary tract infections [15]. Some sulfonamides were also found to be potent carbonic anhydrase, COX-2 and caspase inhibitors [16-18].
Acetyl cholinesterase (AChE, EC 3.1.1.7) belongs to
serine hydrolases class of enzymes. This enzyme system is accountable
for the termination of acetylcholine at cholinergic synapses. The main
function of AChE is to catalyze the hydrolysis of the neurotransmitter
acetylcholine and termination of the nerve impulse in cholinergic
synapses [19]. The inhibitors of this enzyme are the targets for the treatment of Alzheimer's disease [20].
Biological literature review on sulfonamides displayed that little
modification in the structure of sulfonamides can cause remarkable
changes in biological activity. These outcomes stimulated us to focus on
synthesis of variety of N-alkyl/aralkyl-N-(2,3-dihydrobenzo[1,4]dioxin-6-yl)-4-
methylbenzenesulfonamides. Recent research work was a successful
attempt to synthesize new therapeutic agents for the inhibition of
cholinesterase enzyme.
Experimental
Measurements and materials
All of the essential chemicals/solvents were of
analytical grade and procured from authorized suppliers, Merck and Alfa
Aeser branded. The pre-coated silica gel G-25-UV254 plates
were applied for TLC to monitor the completion of reactions using
various percentages of n-hexane and ethyl acetate as mobile phase. Open
capillary tubes were used in Gallon kamp melting point apparatus to
record the melting points. Developed TLC visualized under 254nm UV lamp
and UV inactive substances were identified with the spray of ceric
sulfate solution. Infrared spectra were noted in KBr pellet on a
Jasco-320-A spectrophotometer. 1H-NMR spectra were recorded by Bruker spectrometer in CDCl3
operating at 400MHz at 25 °C. The chemicals shifts (5) were observed in
ppm and coupling constants (δ) were noted in Hertz (Hz). The
abbreviations used in 1HNMR spectral analysis were;
s=singlet, d=doublet, dd=doublet of doublet, t=triplet, br.t=broad
triplet, q=quartet, quint=quintet, sex=sextet, sep=septet, m=multiplet.
Mass spectra (EIMS) were taken on Finngen Mass Spectrometer.
Synthesis of N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4- methylbenzenesulfonamide (3)
2,3-Dihydro-1,4-benzodioxin-6-amine
(1.22mL;0.01mol;1) and 4-methylbenzenesulfonyl chloride (0.90g; 0.01mol;
2) were poured into a 250ml round bottom flask having 30ml of distilled
water. The pH of the suspension was maintained at 9.0 by introducing
10% Na2CO3 solution at room temperature. The
content of reaction was stirred for 2-3 hours and progress of the
reaction was examined by TLC time to time till single spot confirm the
completion of reaction. The product was obtained by the slow addition of
concentrated HCl at pH 2-3 as brown coloured precipitates. These were
filtered, washed with distilled water and air-dried to afford pure
N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4- methylbenzenesulfonamide (3);
Yield: 82%, IR (KBr, cm-1): vmax: 3284 (N-H stretching), 2970 (C-H stretching of aromatic ring], 1650 C=C stretching of aromatic ring), 1410 (SO2 stretching), 1175 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400MHz, δ in ppm): 9.87 (s, -NH, 1H), 7.58 (d, J=8.4Hz, 2H, H-2' &
H-6'), 7.32 (d, J=8.0Hz, 2H, H-3' & H-5'), 6.68 (d, J=8.4Hz, 1H,
H-8), 6.55 (d, J=2.0Hz, 1H, H-5), 6.50 (dd, J=2.4, 6.5Hz 1H, H-7), 4.14
(s, 4H, H-2 & H-3), 2.33 (s, 3H, H-1''); EI-MS (m/z): 305 (M+; C15H15NO4S), 304 (C15H14NO4S)+, 277 (C13H11NO4S)+, 241 (C15H15NO2)+, 213 (C8H7NO4S)+, 155 (C7H7SO2)+, 150 (C8H8NO2)+,, 135(C8H7O2)+,, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+, 65 (C6H5)+, 51 (C4H3)+.
General procedure for the synthesis of N-substituted derivatives 5a-n
Compound 3 (0.1g; 0.3mmol) solubilised in 10ml of N,
N-dimethyl formamide (DMF) aprotic solvent in 100ml round bottom flask.
Lithium hydride (0.004g) was mixed in the reaction mixture to activate
the reaction followed by stirring for 2-3 hours at room temperature.
Then various alkyl/aralkyl halides (4a-n) were introduced and stirring
was continued for further 3-4 hours. Completion of reaction was assured
by TLC displaying single spot. Then reaction content was quenched with
ice cold distilled water along with vigorous shaking to get the
precipitates of N-alkyl/aralkyl-N-(2,3-dihydro-1,4-benzodioxin-
6-yl)-4-methylbenzenesulfon-amides (5a-n) which were left for some time
undisturbed and collected by the filtration or solvent extraction (using
CHCl3) depending upon the nature of the derived compound.
Spectral analysis
The spectral analysis of 5b, 5g, 5h, 5j and 5l has already been reported by our group [21] while that of other compounds is given hereby.
N-(2-Chloroethyl)-N-(2,3-dihydro-1,4-benzodioxin-6- yl)-4-methylbenzenesulfonamide (5a): Off white powder; Yield: 95 %; m.p: 142 °C; Molecular formula: C17H18ClNO4S; Molecular weight: 371g mol-1; IR (KBr, cm-1): vmax: 2976 (C-H stretching of aromatic ring), 1657 (C=C stretching of aromatic ring), 1395 (-SO2 stretching), 1140 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400MHz, δ in ppm): 5 7.50 (d, J=8.5Hz, 2H, H-2' & H-6'), 7.40 (d,
J=8.4Hz, 2H, H-3' & H-5'), 6.66 (d, J=7.6Hz, 1H, H-8), 6.50 (dd,
J=2.5, 8.2Hz, 1H, H-7), 6.42 (d, J=2.6Hz, 1H, H-5),4.28 (br.s, 4H, CH2-2 & CH2-3), 3.60 (t, J=6.5Hz, 2H, CH2-2"), 3.46 (t, J=7.4Hz, 2H, CH2-1"), 2.34 (s, 3H, CH3-7'); EI-MS (m/z): 371 (M+ C17H18CINO4S), 340 (C15H14ClNO4S)+, 304 (C15H14NO4S)+, 300 (C12H11ClNO4S)+,, 237 (C12H11ClNO2)+, 213 (C10H11ClNO2)+, 155 (C7H7SO2+, 135(C8H7O2)+, 107(C6H3O2)+, 91(C7H7)+, 81(C4HO2)+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-N-(2-Iodoethyl)- 4-methylbenzenesulfonamide (5c): Greyish white solid; Yield: 95%; m.p: 132 °C; Molecular formula: C17H18INO4S; Molecular weight: 459g mol-1; IR (KBr, cm-1): vmax:
2989 (C-H stretching of aromatic ring), 1660 (C=C stretching of
aromatic ring), 1379 (-SO2 stretching), 1168 (C-O-C stretching of
ether); 1H-NMR (CDCl3, 400MHz, δ in ppm): 5 7.50
(d, J=7.4Hz, 2H, H-2' & H-6'), 7.42 (d, J=8.5Hz, 2H, H-3' &
H-5'), 6.68 (d, J=6.68Hz, 1H, H-8), 6.54 (d, J=2.5Hz, 1H, H-5), 6.40
(dd, J=2.5, 8.5Hz, 1H, H-7), 4.26 (br.s, 4H, CH2-2 & CH2-3), 3.72 (t, J=7.5Hz 2H, CH2-1''), 3.36 (t, J=6.8Hz, 2H, CH2-2"), 2.36 (s, 3H, CH3-7'); EI-MS (m/z): 459 [M+ C17H18IO4S], 431 (C15H14INO4S)+, 395 (C17H18INO2)+, 368(C10HuINO4S)+,, 326 (C10H10INO2)+, 304 (C15H14NO4S)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4-methyl-N-(1-propyl)benzenesulfonamide (5d): Dark brown powder; Yield: 88%; mp: 136 °C; Molecular formula: C18H21NO4S; Molecular weight: 347g mol-1; IR (KBr, cm-1): vmax: 2988 (C-H stretching of aromatic ring), 1662 (C=C stretching of aromatic ring), 1374 (SO2 stretching), 1148 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400MHz, δ in ppm): 5 7.46 (d, J=8.2Hz, 2H, H-2' & H-6'), 7.34 (d,
J=8.5Hz, 2H, H-3' & H-5'), 6.68 (d, J=7.5Hz, 1H, H-8),6.40 (d,
J=2.5Hz, 1H, H-5), 6.22 (dd, J=2.6, 8.4Hz, 1H, H-7), 4.28 (br.s, 4H, CH2-2 & CH2-3), 3.10 (t, J=7.5Hz, 2H, CH2-1''), 2.34 (s, 3H, CH3-7'), 1.60-1.56 (m, 2H, CH2-2''), 1.05 (t, J=7.4Hz, 3H, CH3-3''); EI-MS (m/z): 347 (M+ C18H21NO4S), 319 (C16H17NO4S)+, 304 (C15H14NO4S)+, 283 (C18H21No2)+, 256 (C11H14NO4S)+, 178 (C11H14NO2)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4-methyl-N-(1-pentyl)benzenesulfonamide (5e): Brown powder; Yield: 85%; m.p: 114 °C; Molecular formula: C20H25NO4S; Molecular weight: 375g mol-1; IR (KBr, cm-1): vmax: 2984 (C-H stretching of aromatic ring), 1678 (C=C stretching of aromatic ring), 1376 (SO2 stretching), 1142 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400 MHz, δ in ppm): 5 7.40 (d, J=8.4Hz, 2H, H-2' & H-6'), 7.34 (d,
J=8.5Hz, 2H, H-3' & H-5'), 6.66, (d, J=7.8Hz, 1H, H-8), 6.44 (dd,
J=2.7, 8.5Hz, 1H, H-7), 6.28 (d, J=2.6Hz, 1H, H-5), 4.28 (br.s, 4H, CH2-2 & CH2-3), 3.20 (t, J=7.5Hz, 2H, CH2-1''), 2.34 (s, 3H, CH3-7'), 1.40-134 (m, 6H, CH2-2" to CH2-4"), 0.90 (t, J=7.6Hz, 3H, CH3-5"); EI-MS (m/z): 375 (M + C20H25NO4S), 347 (C18 H21NO4S)+, 311 (C20H25NO2)+, 304 (C15H14NO4S)+, 284 (C13H18NO4S)+, 220 (C13H18NO2)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+, 71 (C5H2+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4-methyl-N-(2-pentyl)benzenesulfonamide (5f): Brown solid; Yield: 92%; m.p: 124 °C; Molecular formula: C20H25NO4S;
Molecular weight: 375g mol-1; IR (KBr, cm-1): vmax: 2994 (C-H
stretching of aromatic ring), 1660 (C=C stretching of aromatic ring),
1372 (-SO2 stretching), 1144 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400MHz, δ in ppm): 57.44 (d, J=8.5Hz, 2H, H-2' & H-6'), 7.30 (d,
J=8.6Hz, 2H, H-3' & H-5'), 6.64, (d, J=8.6Hz, 1H, H-8), 6.54 (dd,
J=2.6, 8.8Hz, 1H, H-7), 6.30 (d, J=2.5Hz, 1H, H-5), 4.28 (br.s, 4H, CH2-2 & CH2-3), 2.84-2.80 (m, 1H, H-2"), 2.34 (s, 3H, CH3-7'), 1.51-1.50 (m, 2H, CH2-3"), 1.34 (Sext., J=7.4Hz, 2H, CH2-4"), 0.90 (t, J=7.5Hz, 3H, CHr5"); EI-MS (m/z): 375 (M+; C20H25NO4S), 347 (C18 H21NO4S)+, 311 (C20H25NO2)+, 304 (C15H14N04S)+, 284(C13H18NO4S)+, 220 (C13H18NO2)+, 155 (C7H7SO2)+, 135 (C8H7O2)+,107 (C6h3O2+, 91 (C7H7)+, 81 (C4HO2)+, 71 (CSHU)+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4-methyl-N-(2-methybenzyl)benzenesulfonamide (5i): Brown powder; Yield: 88%; m.p: 160 °C; Molecular formula: C23H23NO4S; Molecular weight: 409g mol-1; IR (KBr, cm-1): vmax: 2998 (C-H stretching of aromatic ring), 1680 (C=C stretching of aromatic ring), 1374 (-SO2 stretching), 1162 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400 MHz, δ in ppm): 5 7.44 (d, J=8.4Hz, 2H, H-2' & H-6'), 7.38 (d,
J=7.7Hz, 2H, H-3' & H-5'), 7.34-7.28 (m, 4H, H-3'' to H-6''), 6.60,
(d, J=7.5Hz, 1H, H-8), 6.50 (dd, J=2.5, 8.5Hz, 1H, H-7), 6.25 (d,
J=2.6Hz, 1H, H-5), 4.38 (s, 2H, CH2-7''),4.28 (br.s, 4H, CH2-2 & CH2-3), 2.34 (s, 3H, CH3-7'), 2.26 (s, 3H, CHr8"); EI-MS (m/z): 409 (M+; C23H23N04S), 381 (C21H19NO4S)+, 345 (C23H23NO2)+, 318 (C16H16NO4S)+, 304 (C15H14NO4S)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 105 (C8H9)+, 107 (C6H3O2)+, 91(C7H7)+, 81 (C4HO2)+.
N-(3-Chlorobenzyl)-N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-methylbenzenesulfonamide (5k): Grey powder; Yield: 96%, m.p: 152 °C; Molecular formula: C22H20ClNO4S; Molecular weight: 429gmol 1; IR (KBr, cm-1): vmax: 3010 (C-H stretching of aromatic ring), 1672 (C=C stretching of aromatic ring), 1382 (-SO2 stretching), 1090 (C-O-C stretching of ether); 1H-NMR (CDCl3,
400 MHz, δ in ppm): 5 7.50 (br.s, 1H, H-2"), 7.42 (d, J=8.8Hz, 2H, H-2'
&H-6'), 7.36 (d, J=8.5Hz, 2H, H-3' & H-5'), 7.30-7.25 (m, 3H,
H-4" to H-6'"), 6.66, (d, J=6.5Hz, 1H, H-8), 6.52 (dd, J=2.6, 8.5Hz, 1H,
H-7), 6.18 (d, J=2.6Hz, 1H, H-5), 4.42 (s, 2H, CH2-7''), 4.28 (br.s, 4H, CH2-2 & CH2-3), 2.36 (s, 3H, CH3- 7'); EI-MS (m/z): 429 (M+; C22H20ClNO4S), 402 (C20H16ClNO4S)+, 365 (C22H20ClNO2)+, 338 (C15H13ClNO2)+, 304 (C15H14N04S)+, 274 (C15H13ClNO2)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 171 (C7H6Cl)+, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+.
N-(2-Bromobenzyl)-N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-methylbenzenesulfonamide (5m): Brown powder; Yield: 96%; m.p: 168 °C; Molecular formula: C22H20BrNO4S; Molecular weight: 475gmol-1; IR (KBr, cm-1):
vmax: 3010 (C-H stretching of aromatic ring), 1670 (C=C stretching of
aromatic ring), 1366 (-SO2 stretching), 1156 (C-O-C stretching of
ether); 1H-NMR (CDCl3, 400MHz, δ in ppm): 57.50 (br.d,
J=6.8Hz, 1H, H-3''), 7.42 (d, J=8.5Hz, 2H, H-2' &H-6'), 7.36 (d,
J=8.5Hz, 2H, H-3' & H-5'), 7.28-7.24 (m, 4H, H-4'' to H-6"), 6.62,
(d, J=6.8 Hz, 1H, H-8), 6.48 (dd, J=2.5, 8.6Hz, 1H, H-7), 6.20 (d,
J=2.8Hz, 1H, H-5),4.41 (s, 2H, H-7"), 4.28 (br.s, 4H, CH2-2 & CH2-3), 2.34 (s, 3H, CH3- 7'); EI-MS (m/z): 475 [M+; C22H20BrNO2], 447 (C20H16BrNO4S)+, 411 (C22H20BrNO2)+, 320 (C15Hl3BrN0l)+, 304 (C15H14NO4S)+, 384 (C15H13BrNO4S)+, 171 (C7H6Br)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 107 (C6H0l)+, 91 (C7H7)+.
N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-4-methyl-N-(4-fluorobenzyl)benzenesulfonamide (5n): Light grey powder; Yield: 90%, m.p: 122 °C; Molecular formula: C22H20FN04S; Molecular weight: 413g mol 1; IR (KBr, cm-1):
vmax: 2994 (C-H stretching of aromatic ring), 1686 (C=C stretching of
aromatic ring), 1380 (-SO-, stretching), 1195 (C-F stretching), 1086
(C-O-C stretching of ether); 1H-NMR (CDCl3, 400MHz, δ in
ppm): 57.44 (d, J = 8.6Hz, 2H, H-2' & H-6'), 7.34 (d, J = 7.5Hz, 2H,
H-3' & H-5'), 7.38 (dd, J=5.3, 8.5Hz, 2H, H-2” & H-6”,
multiplicity due to coupling of F19), 7.14 (br.t, J=8.6, 2H, H-3” &
H-5”, due to coupling of F19), 6.62, (d, J=7.5Hz, 1H, H-8), 6.50 (dd,
J=2.5, 7.8Hz, 1H, H-7), 6.20 (d, J=2.5Hz, 1H, H-5), 4.36 (s, 2H, CHr7"),4.28 (br.s, 4H, CH2-2 & CH2-3), 2.32 (s, 3H, CH3-7'); EI-MS (m/z): 413 (M+; C22H20FNO4S), 385 (C20H16FNO4S)+, 349 (C22H20FNO2)+, 322 (C15H13FNO4S)+, 304 (C15H14NO4S)+, 155 (C7H7SO2)+, 135 (C8H7O2)+, 109 (C7H6F)+, 107 (C6H3O2)+, 91 (C7H7)+, 81 (C4HO2)+, 65 (C6H5)+, 51(C4H3)+.
Acetylcholinesterase inhibition assay
The inhibition activity of acetylcholinesterase was performed by according to a reported method [22] with little modifications. Total volume of the reaction mixture was 100μL and it also contained 60μL Na2HPO4,
which acts as buffer having 50mm concentration (pH 7.7). Ten μL test
compound 0. 5mm well-1, followed by the addition of 10μl (0.5 unit
well-1) enzyme. The reaction contents was agitated well and read prior
at the wavelength 405nm. Then mixture was pre-incubated at 37 °C for 10
minute. The reaction was started by adding 10μl of 0.5mm well-1
substrate (acetylthiocholine iodide) followed by the addition of 10μl
DTNB (0.5mm well-1). After 15 min of incubation at 37 °C, absorbance was
recorded at 405nm using 96-well plate reader Synergy HT (BioTek, USA).
Each and every experiment was carried out with their particular controls
in triplicate. Eserine (0.5mm well-1) was act as positive control. The
equation that was used to calculate % inhibition was:
Inhibition (%)=(Control-Test)/Controlx100
IC50 values (concentration at which there
is 50% enzyme inhibition) of compounds were calculated using EZ-Fit
Enzyme kinetics software (Perella Scientific Inc. Amherst, USA).
Statistical analysis
All the measurements were done in triplicate and
statistical analysis was performed by Microsoft Excel 2010. Results are
presented as mean ± SEM.
Results and Discussion
Chemistry
The synthesis of various derivatives, 5a-n, derived
by the N-substitution of N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-
methylbenzenesulfonamide (3) has been outlined in Scheme 1 and Table 1.
All methods and conditions for this research work are mentioned in
experimental section. The synthesis was initiated by the reaction of
N-2,3-dihydro-1,4-benzodioxine-6-amine (1) with 4-methylbenzenesulfonyl
chloride (2) in the presence of 10% Na2CO3 at
adjusted pH 9 under constant stirring for 2-3 hours at room temperature
to yield the parent sulfonamide, N-(2,3-
dihydro-1,4-benzodioxin-6-yl)-4-methylbenzenesulfonamide (3). Then,
N-alkylation/aralkylation of this parent 3 was carried out with
different alkyl/aralkyl halides (4a-n) in DMF as a polar aprotic solvent
and LiH as the base to yield the target compounds, 5a-n. We have
already reported 5b, 5g, 5h, 5j and 5l along with their structural
characterizations [21],
however, we are reporting other compounds as new molecules in this
investigation. The structures of the studied molecules were deduced
through IR, 1H-NMR and EI-MS spectral techniques. For the
expediency of the readers, one of the compounds is discussed hereby in
detail. The molecule 5f was obtained as brown solid having melting point
124 °C. The molecular formula C20H25NO4S
of this molecule was established by its EI-MS showing the molecular ion
peak at m/z 375 and by counting the number of protons in its 1H-NMR
spectrum. The subsequent fragmentation peaks in its EI-MS spectrum also
supported this assignment. The IR spectrum showed absorption bands at
2994, 1660, 1372 and 1144 for the C-H stretching of aromatic ring, C=C
stretching of aromatic ring, SO2 and C-O-C stretching respectively. In
its 1H-NMR spectrum, the presence of a
4-methylbenzenesulfonyl moiety was assured by two ortho-coupled doublets
at 5 7.44 (d, J = 8.5Hz, 2H, H-2', H-6') and 7.30 (d, J = 8.6Hz, 2H,
H-3', H-5') along with a singlet at 5 2.34 (s, 3H, CH3-7')
for methyl group. The 2,3-dihydro-1,4- benzodioxin-6-yl moiety in this
molecule was corroborated by an otho-coupled doublet at 5 6.64 (d,
J=8.6Hz, 1H, H-8), a meta-coupled doublet at 5 6.30 (d, J=2.5Hz, 1H,
H-5) and corresponding doublet of doublet at 5 6.54 (dd, J=2.6, 8.8Hz,
1H, H-7) along with a broad singlet at 5 4.28 (br.s, 4H, CH2-2 & CH2-
3) for two oxygenated methylenes. The N-substituted 2-pentyl group in
this molecule was characterized by its typical signals in aliphatic
region at 5 2.84-2.80 (m, 1H, H-2"), 1.51-1.50 (m, 2H, CH2-3"), 1.34 (Sext., J=7.4Hz, 2H, CH2-4"), and 0.90 (t, J=7.5Hz, 3H, CH3-5").
So, on the basis of above collected evidences, the structure of 5f was
named as N-(2,3-dihydro-1,4-benzodioxin-6- yl)-4-methyl-N-(2-pentyl)
benzenesulfonamide. In an analogous manner, the structures of other
derivatives of the series were characterized [22].
Acetylcholinesterase inhibition
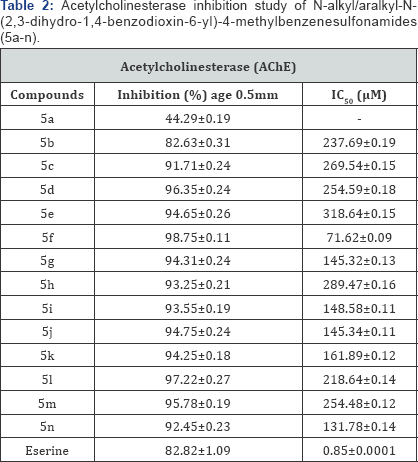
The screening of all the derivatives 5a-n, against
acetylcholinesterase (AChE) enzyme demonstrated that all the molecules
of the series were active, except 5a. These molecules exhibited moderate
to weak inhibitory potential and the results are tabulated in Table 2 in the form of % age inhibition and IC50 values. Among these molecules, 5f, was found to be better inhibitor against this enzyme having IC50
value of 71.62±0.09μM, probably due to the substitution of a branched
aliphatic group i. e. 2-pentyl group. The molecule, 5n, having
substitution of 4-fluorobenzyl group, also showed notable inhibitory
potential with IC50 value of 131.78±0.14μM. An extremely potent, eserine molecule was used as reference standard in this assay which has an IC50 value of 0.85±0.0001μM.
Conclusion
The targeted derivatives, 5a-n, were synthesized in
good yields with a facile method and some of them exhibited a notable
inhibitory potential against acetyl cholinesterase enzyme, therefore,
these molecules might find their utility as possible therapeutic agents
for the treatment of Alzheimer's disease.
Acknowledgement
Special thanks are paid to the Higher Education Commission (HEC) of Pakistan for financial grant to execute this study.
please click on: https://juniperpublishers.com/napdd/index.php


Comments
Post a Comment