Landscaping of Quinoline based Heterocycles as Potential Antimicrobial Agents: A Mini Review-Juniper Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
In this mini-review, we have compiled our research
group's prior efforts aimed at developing potential, novel and safe
antimicrobial agents with improved efficiency by using the hybrid
molecular approach on quinoline hybrids. Present mini-review article
deals with landscaping of quinoline based bioactive analogues with
pyrazoles, thiazoles, 4-thiazolidinones, pyrimidines and 2-pyridones
heterocycles. Quinoline motifs containing hybrids with two or more fold
antimicrobial activity are described hereby. We have reported a mini
review of 174 compounds prepared in our laboratory. The SAR of the most
promising compounds is also discussed.
Keywords: Quinoline; Antimicrobial agents; Pyrazole; 4-Thiazolidinone; 2-Pyridone Abbrevations: AC: Aspergillus Clavatus; An: Aspergillus Niger; Ca: Candida Albicans; Ec: Escherichia Coli; Pa: Pseudomonas Aeruginosa; Sa: Staphylococcus Aureus; Sp: Streptococcus Pyogenes
Introduction
The ever swelling incidences of infectious
resistance pose a major threat to the scientific society and thus the
basic requirement for discovery and development of newer and safer
anti-microbial agents with novel mode of action is becoming acute [1].
One of the best ways to tackle this type of enormous problem is to
synthesize hybrid molecules by uniting two or more bioactive
heterocyclic moieties in a single molecular framework. Libraries of
privileged heterocyclic structures can be used in place of modified
drugs. In continuation to this, the design of molecules is made in such a
way that may have lower adverse effects with shorter treatment regimens
[2].
Quinoline is a heterocyclic aromatic organic compound having molecular formula C9H7N, possessed by a double-ring structure containing a benzene ring fused to pyridine at two adjacent carbon atoms [3].
Quinoline is a core pharmacophore in the recently developed anti
tubercular drugs bedaquiline (TMC207). This is the first drug with a
novel mechanism of action for TB in more than 40 years. Bedaquiline a
diarylquinoline is the sole drug specifically indicated to treat MDR-TB [4].
It works via inhibition of enzyme adenosine triphosphate (ATP)
synthase, which is the energy source for the bacterium. Furthermore,
quinoline moiety exhibits biological activities like antibacterial [5], anti-malarial [6], anti-TB [7], anti-proliferative [8] and anti cancer [9].
*MIC (in μg/mL) of standard drug Ampicillin against: Ec (MTCC-443): 100; Pa (MTCC-1688): 100; Sa (MTCC-96): 250; Sp (MTCC 442): 100 & Griseofulvin against: An (MTCC-282): 100; Ac (MTCC-1323): 100.
According to literature survey, more potent novel
chemical entities can be envisaged by uniting two or more heterocyclic
systems into a single molecule [10].
With the aim of developing new affordable antimicrobials having new
mode of action, our research group is actively involved in developing
novel hybrid heterocycles and screen them for antimicrobial activity
with some more promising results. We have further modified active
molecules to develop compounds with more potency and less toxicity. In
this article we report here published results of our effort towards
development of more potential antimicrobial agents. Since a couple of
years our research group has published several important findings for
the benefit of scientific community on quinoline based heterocycles.
Previously we have published review articles of our own findings which
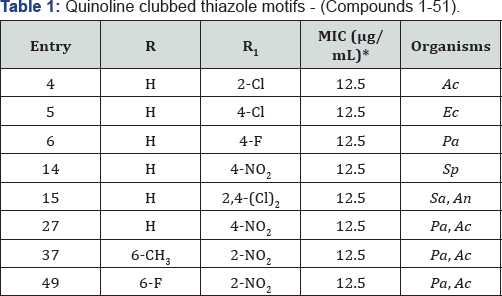
received very well appreciation by scientific community [11,12] (Table 1).
Quinoline clubbed pyrazole motifs - (Compounds 1-51)
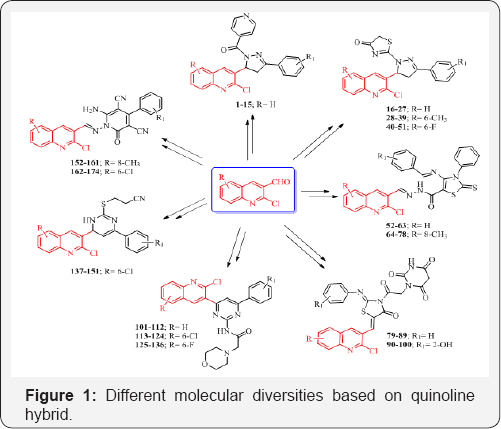
Looking at the versatile biological portfolios of
quinoline; pyrazole with 4-thiazolidinones and pyridine heterocyclic
ring systems, new structural hybrids of three moieties were prepared and
subjected to antimicrobial activity [13-15]. Quinoline-pyrazole hybrids 1-15 and 16-51 are shown in Figure 1. Compounds 1-15 substituted with H at R and 2-Cl, 4-Cl, 4-F and 4-NO2 at R1
position, compounds 4, 5, 6 and 14 came out as most potent
antimicrobial agents with MIC of 12.5μg/ mL against various bacterial
and fungal strains. Compound 15 displayed excellent antibacterial and
antifungal activities against Staphylococcus aureus and Aspergillus niger with MIC of 12.5μg/mL. From the series of compounds 16-51, compound 27 (R=H and R1=4-NO2) stood out as most promising antibacterial as well as antifungal potency with MIC of 12.5μg/mL against P aeruginosa and A. clavatus respectively. Nitro group at ortho position in compounds 37 (R=6-CH3) and 49 (R=6-F) were identified as a most active antimicrobial agents with MIC of 12.5μg/mL against P. aeruginosa and A. clavatus strains taking Ampicillin and Griseofulvin as standard drugs.
Quinoline clubbed thiazole motifs - (Compounds 52–78)
A series of quinoline clubbed thiazole motifs were synthesized and subjected to antimicrobial evaluation [16,17].
Hybrid structure is depicted in Figure 1. Compound 59 (R=H) containing 3,4,5-(OCH3)3 group showed excellent inhibition (25μg/mL) against P aeruginosa bacterial strain as compared to the standard drug Gentamycin. Compounds 74 and 78 (R= 8-CH3)
containing electron withdrawing substituents such as -Cl and -F
exhibited good antimicrobial activity. It is a well-known fact that
electron withdrawing substituents increase the lipophilicity of
molecules. This property is directly related to antimicrobial activity
as it helps the compound to diffuse through biological membranes and
reach the site of action (Table 2).
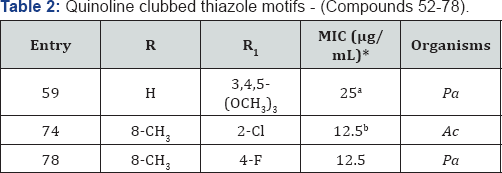
*aMIC (in μg/mL) of standard drug Gentamycin against: Pa (MTCC- 1688): 0.5
*bMIC (in μg/mL) of standard drug Ampicillin against: Pa (MTCC- 1688): 100 & Griseofulvin against: Ac (MTCC-1323): 100.
Quinoline clubbed 4-thiazolidinone motifs - (Compounds 79-100)
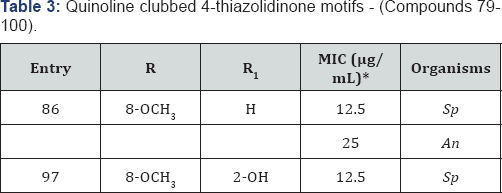
*MIC (in μg/mL) of standard drug Ampicillin against: Sp (MTCC 442): 100 & Griseofulvin against: An (MTCC-282): 100.
Owing to the pharmacological importance of
4-thiazolidinones and quinoline, we synthesized new quinoline
derivatives containing 4-thiazolidinone moiety and screened them for
antimicrobial activity [18-19].
Quinoline containing 4-thiazolidinone structural hybrids were shown in
Figure 1 (Compounds 79-100). Compound 86 substituted with 8-OCH3 at R and H at R1 position displayed MIC of 12.5 and 25μg/ mL against S. pyogenes and A. niger respectively. Compound 97 exhibited maximum antibacterial inhibition (12.5μg/mL) against S. pyogenes using Ampicillin as standard drug. SAR studies revealed that substitution on quinoline ring at 8th position by an electron releasing groups seems to be better than that of electron withdrawing substitutions (Table 3).
Quinoline clubbed pyrimidine motifs - (Compounds 101-151)
Pyrimidine analogues play a vital role in drug
discovery and its development process, and have significant
pharmacological reputation. In view of broad spectrum biological
activities of quinolines and pyrimidines, we designed and synthesized
new prototypes of quinoline and pyrimidine nucleus in a single molecular
framework and successively evaluated them for antimicrobial activity [20-22]. Structures of quinoline pyrimidine hybrid are represented in Figure 1 (Compounds 101-136 and 137-151). Compound 112 (R=H and R1= 4-NO2) demonstrated the most potent antibacterial activity with MIC of 12.5 μg/mL against E. coli and S. pyogenes. Compounds 121 (R=6-Cl and R1=4-F) and 122 (R= 6-Cl and R1=2-NO2)
were identified as the most potent antifungal as well as antibacterial
agents with MIC of 12.5μg/mL, respectively. Compound 131 (R= 6-F and R1=2-F) exhibited maximum inhibition against E. coli.
It was observed on the basis of SAR studies that high antimicrobial
properties of these compounds may be attributed to the presence of
electron withdrawing substituents such as nitro and fluoro at phenyl
ring of pyrimidine nucleus. On the other hand, compounds 147 and 149
contain electron donating substituents at para position showed 12.5μg/mL MIC against Staphylococcus pyogenes and Staphylococcus aureus. In-vitro cytotoxicity of most active antimicrobial compounds 147 (R=6-Cl and R1=4-OH) and 149 (R=6-Cl and R1=4-OCH3)
were evaluated against human cervical cancer cell line (HeLa) by MTT
colorimetric assay and the tested compounds exhibited any significant
cytotoxic effect on HeLa cells, suggesting a great potential for their in-vivo use as antimicrobial agents (Table 4).
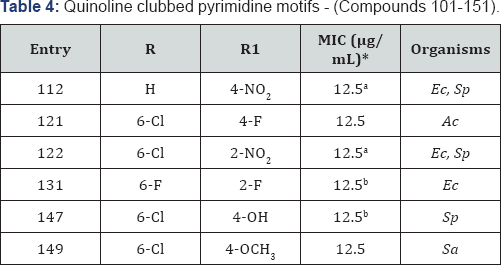
*aMIC (in μg/mL) of standard drug Ampicillin against: Ec (MTCC-443): 100; Sp (MTCC 442): 100
*bMIC (in μg/mL) of standard drug Ciprofloxacin against: Ec (MTCC- 443): 25; Sp (MTCC 442): 50; Sa (MTCC-96): 50 & Griseofulvin against: Ac (MTCC-1323): 100.
Quinoline clubbed 2-pyridone motifs - (Compounds 152-174)
Rich biological silhouette of quinoline and
2-pyridone analogues gave us an impetus to design, synthesis and
evaluate new structural hybrids of both these nucleus in single platform
[23,24]. Quinoline-2-pyridone hybrid is displayed in Figure 1 with compound 152-174. Compound 153 endowed with 4-CH3 substituent revealed remarkable MIC of 62.5μg/mL against bacterial strain of E. coli as compared to the standard drug Gentamycin in. From antibacterial activity data, it was observed that compounds 163 (R=6-Cl and R1=4-CH3) and 164 (R=6-Cl and R1=4-OCH3)
were identified as most active compounds (MIC=12.5μg/mL) against
various Gram-negative as well as Gram-positive bacterial strains. 3-OH
analogue possessed maximum inhibition at 12.5μg/mL against Candida albicans fungal strain (Table 5).
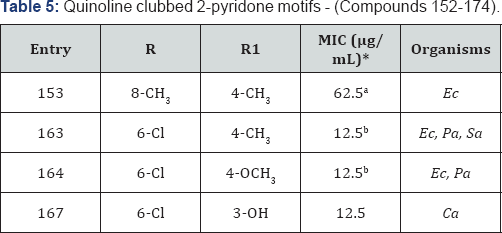
*aMIC (in μg/mL) of standard drug Gentamycin against: Ec (MTCC-443): 0.25
*bMIC (in μg/mL) of standard drug Ciprofloxacin against: Ec (MTCC-443): 25; Pa (MTCC 1688): 25; Sa (MTCC-96): 50 &
Griseofulvin against: Ca (MTCC-227): 500.
Conclusion
Current article discusses the principle idea for
co-relating the structures of potent heterocyclic molecules and
antimicrobial activity. The quinoline based molecule classes reported
here are pyrazoles, thiazoles, 4-thiazolidinones, pyrimidines and
2-pyridones. Our endeavours to design, synthesis and examine novel
quinoline based structural hybrids of various bioactive heterocycles as
potential antimicrobial agents have yielded promising results in primary
biological evaluation. In our forthcoming efforts, we will assess these
structural hybrids and their modified derivatives for further in-vivo
studies. The most potent compounds will be validated against various
available molecular targets with the goal to produce novel antimicrobial
agents with superior remedial effect, minor toxicity and less side
effects.
Acknowledgement
Authors are gratified to the UGC, New Delhi and
Department of Science and Technology, New Delhi (DST-FIST-SR/FST/CSI-
212/2010) for financial support under the NON-SAP and DST FIST programs,
respectively. Dr. Bonny Y Patel is thankful to CSIR (CSIR EMR-II
02(0188)14/EMR-II), New Delhi for CSIR EMR-II Research Associate-I and
Mr. Krunalsinh A Jadeja is thankful to UGC, New Delhi for UGC-MRP
Project Fellowship (UGC F. No.-43- 164/2014(SR)).


Comments
Post a Comment