Mechanistic Aspects of Autism Involving Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Pollutants, Antioxidants, Cell Signaling and Genes -Open Access Publishers
Novel Approaches in Drug Designing & Development (NAPDD)
Autism is a center of attention both in research and
the media. This review is mainly concerned with mechanistic involvement
of electron transfer, reactive oxygen species, oxidative stress and
antioxidants, in addition to cell signaling and genetic aspects.
Pollution from the environment is a factor. The most sensitive stage is
the fetus. The neuronal network in the brain plays an important role.
Antioxidants can act in prevention or amelioration. Interaction between
the various aspects is addressed. The effect of vaccine is discussed.
Keywords: Autism; Electron transfer; Radicals; Oxidative stress; Pollutants; Antioxidants; GenesAbbrevuations: ET: Electron Transfer; ROS: Reactive Oxygen Species; OS: Oxidative Stress; AO: Anti Oxidant
Introduction
Autism has attracted much attention in recent years,
both in research and in the media. This review summarizes evidence for
important involvement of electron transfer (ET), reactive oxygen species
(ROS), oxidative stress (OS), antioxidants (AOs) and genes. Evidence
points to a key role for pollution. The fetus is the stage most
susceptible to damaging effects, mostly to the brain and central nervous
system. The unifying mechanistic theme has been widely applied
previously as set forth as follows:
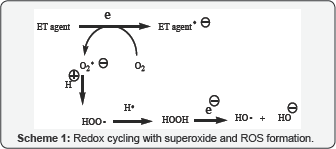
"The preponderance of bioactive substances, usually
as the metabolites, incorporate ET functionalities. We believe these
play an important role in physiological responses. The main group
include quinones (or phenolic precursors), metal complexes (or
complexors), aromatic nitro compounds (or reduced hydroxylamine and
nitroso derivatives), and conjugated imines (or iminium species).
Resultant redox cycling is illustrated in (Scheme 1). In vivo
redox cycling with oxygen can occur, giving rise to oxidative stress
(OS) through generation of reactive oxygen species (ROS), such as
hydrogen peroxide, hydro peroxides, alkyl peroxides, and diverse
radicals (hydroxyl, alkoxyl, hydro peroxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform catalytically in the reduction of O2.
In some cases ET results in involvement with normal
electrical effects (e.g., in respiration or neurochemistry). Generally,
active entities possessing ET groups display reduction potentials in the
physiologically responsive range, (i.e., more positive than about -0.5
V). Hence, ET in vivo can occur resulting in production of ROS
which can be beneficial in cell signaling at low concentrations, but
produce toxic results at high levels. Electron donors consist of
phenols, N-hetero cycles or disulfides in proteins which produce
relatively stable radical cations. ET, ROS and OS have been increasingly
implicated in the mode of action of drugs and toxins, (e.g.,
anti-infective agents [1], anticancer drugs [2], carcinogens [3], reproductive toxins [4], nephrotoxins [5], hepatotoxins [6], cardiovascular toxins [7], nerve toxins [8],mitochondrial toxins [8], abused drugs [9], pulmonary toxins [10] ototoxins [11] and various other categories [12].
There is a plethora of experimental evidence supporting the ET-ROS theoretical framework [1-12].
This evidence includes generation of the common ROS, lipid per
oxidation, degradation products of oxidation, depletion of AOs, effect
of exogenous AOs, and DNA oxidation and cleavage products, as well as
electrochemical data. This comprehensive, unifying mechanism is
consistent with the frequent observation that many ET substances display
a variety of activities (e.g., multiple-drug properties), as well as
toxic effects. It is important to recognize that mode of action in the
bio domain is often involved with many physiological actions and is
multifaceted. In addition to the ET- ROS-OS approach, other aspects may
pertain, such as, enzyme inhibition, allosteric effects, receptor
binding, metabolism and physical factors. A specific example involves
protein binding by quinones in which protein and nucleophiles, such as
amino or thiol, effect conjugate addition" [13].
Pollution and ET-ROS-OS
A recent study deals with effect of air pollution on rates of autism in different locations [14,15].
The rate in California was related to that in North Carolina involving
similar environmental chemical pollution. A similar study showed autism
spectrum disorders in relation to distribution of hazardous air
pollutants in the San Francisco Bay area [16].
The temporal trends in autism for birth years 1970-2005 were studied.
Autism prevalence has risen dramatically in the U.S over the last
several decades and this effect is correlated to environmental factors [17].
Also, women in the third trimester of pregnancy were more susceptible
to the damaging effects of air pollution on the fetus. Focus of the
research was on exposure to coarse and fine particulate matter in the
air. Results are consistent with the theory involving links between
autism and altered brain neuro development, specifically synaptic
connections.
A recent, relevant review involves the unifying
mechanism of ET-ROS-OS. An investigation revealed perinatal exposure to
the highest and lowest quintile of diesel, lead, manganese and cadmium,
and an overall measure of metals were significantly associated with
autism spectrum disorders [18]. Autism is also related to toxicity of poly nuclear aromatic nitro compounds present in pollutants from engine exhausts [19].
The report provides a mechanistic rationale at the molecular level for
the adverse effects. As indicated in the Introduction, aromatic nitro
compounds comprise one of the classes of ET agents. Generally, the
nitroso metabolite serves as a better agent for ET leading to redox
cycling with generation of ROS-OS.
The literature contains numerous reports dealing with
involvement of ROS-OS in autism. The articles involve various aspects
of the association. A review on physiological abnormalities in autism
places focus on immune dysregulation, OS, inflammation, environmental
toxicity and mitochondrial dysfunction [20]. Autistic children exhibit higher urinary levels of aspartame which is an indication of lipid per oxidation [21].
Levels of per oxidation correlated with vascular biomarker ratios.
Hence, increased OS could play a role in autism development and
manifestations. Results indicate that bisphenol A, an important
industrial chemical, induces enhanced OS and mitochondrial dysfunction
in autism [22]. A review presents a relation between phenol and toxicity based on ET-ROS-OS [23]. Genetic predisposition and environmental poisons have been associated with autism [24].
Enhanced concentrations of 3-nitrotyrosine, an indicator of OS, are
present in the cerebella of autistics. Findings indicate brain changes
in the OS marker. Camel milk, an AO, is a potential therapeutic agent in
autism [25].
There is decrease in OS by AO enzymes and other AO molecules. ROS are
known to be involved in many neuro psychiatric disorders [26].
NO and other agents related to OS may play a role. An article raises
the question: could oxidative stress (OS) from psychosocial stress
affect neurodevelopment in autism? [27]. There is increasing support for involvement of OS and signaling in autism [28].
An up regulation of signaling results in disturbance of OS homeostasis
that leads to increased risk of autism. Autistic children are more
susceptible to OS in the form of enhanced lipid per oxidation and
deficiency of AOs [29].
There may be benefit from AO supplementation. AO enzymes, SOD and GSH
peroxidase, were employed. Levels of malondialdehyde, an indication of
lipid per oxidation, were measured. Early data on AO status may lead to
less OS before brain injury can occur. Studies reveal that autism is
associated with OS, mitochondrial dysfunction, inflammation and immune
dysregulation [30]. ROS production and elevated OS are present in autism [31].
The condition is responsible for damage to mitochondrial DNA. A
combination of genetic and epigenetic factors in utero leads to DNA
alterations similar to that in older individuals. The importance of
external factors, including environmental pollution, is related to
increase in autism [32].
The condition is considered to be an epidemic. Toxic factors, such as
OS, may be responsible for nerve injury to the brain. Multiple forces
may interplay leading to greater vulnerability to OS, toxicity and
neuronal insult. The neuro disorders of autism are believed to be
related to OS arising from ROS, which may be a target for therapy [33].
GSH can serve as an AO for protection against ROS and neuro
inflammation. Decreasing OS could be a treatment. Genetic factors may be
involved in autism, including abnormal genes of OS pathways and
increased OS [34]. In a related report, the relationship of gene polymorphism and OS were studied [35].
Change in iron metabolism in the CNS may be a factor in autism. As
noted in the Introduction, metal compounds, such as Fe, are well known
ET agents. An autism hypothesis is based on a connection between OS and
altered sulfur metabolism [36].
Environmental bacterial contaminants might result in increased OS.
8-OH-dG, a well known product arising from oxidative damage to DNA by
ROS, is present at increased concentrations in autism [37].
The investigation involved cerebella DNA. A report is based on the idea
that there is interaction between genetic and environmental factors
with involvement of OS [38].
A result may be a change in redox status. Lipid per oxidation is
enhanced as part of the scenario. Immune cells in autism exhibit greater
oxidation overall [39].
A deficiency exists in GSH redox/AO capacity. Findings indicate that
loss of the redox homeostasis and chronic OS may lead to immune
dysregulation. In the autistic condition, there are lower levels of AOs,
such as GSH, cysteine and homo cysteine [40]. The condition results in enhanced danger of OS. A related study led to a similar conclusion [41]. Another article deals with a link between OS and erythrocyte membrane alterations[42]. Greater OS in autism is characterized by enhanced free radical production, impaired energetic and higher excitotoxicity [43].
The abnormal brain and gut are more prone to oxidative insult.
Increased red cell lipid peroxides and urine isoprostanes, products of
OS, point to enhanced oxidative attack. Powerful AOs, e.g., vitamin C,
improved the behavior of patients. The benefits appear to be related to
lesson ed OS, an understanding of which should be beneficial. A
mechanism is proposed linking OS in autism with abnormal membrane
lipids, inflammation, altered immune response, excitotoxicity and
changed energy metabolism [44].
These factors play an important role in clinical symptoms and
pathogenesis. A 2005 article deals with a general overview of OS in the
disease [45].
A recent review deals with environmental toxicants and autism [46].
Although a role for genes is widely acknowledged, there is evidence for
equal involvement of environmental pollutants. The toxic materials
include heavy metals, air toxicants, site waste, solvents,
polychlorinated biphenyls, phthalates and pesticides, with the strongest
being pesticides and air pollutants. Genes and environmental toxicants
may act synergistically during neurodevelopment. In utero exposure to
air pollutants was studied in Los Angeles in relation to autism [47].
The pollutants included 1,3-butadiene, formaldehyde, Perchloroethylene,
lead and aromatic solvents, such as xylenes. The conclusion was that
autism in children may increase after in utero exposure to toxic air
materials from industrial emissions and traffic. Oxidative stress was
examined as a factor in autism and possible target in therapy [48].
In recent years, OS has been a focus in the pathogenesis of various
neuropsychiatric disorders including autism. Evidence points to higher
levels of OS and lower levels of AO defenses in the brain. The review
addresses the role of OS and oxidative balance, together with
therapeutic strategies. In a study, a number of autistic children
exhibited a lower level of oxidized GSH, a biomarker of OS, in a clean
room environment [49].
Improvement in 4 of 5 markers of OS was observed. Reduced AO levels
were noted in autistic Chinese children which is a sign of OS [50].
Lowered AO concentrations were recorded for the thiols homocystiene and
GSH. The pathogenesis of autism is often associated with OS in the
brain [51]. Administration of astaxanthin (Figure 1)
proved beneficial and diminished OS in several organs, including the
brain. The extensively conjugated dicarbonyl can act as an AO or a
pro-oxidant. As an AO, the absorbed electron in ET can be donated to an
oxidant or an electron can be provided from the long conjugated system.
As an oxidant, the vinylogous conjugated system could participate in ET
by generating ROS (52).
A cell's resistance to OS depends on genes for RNA in
the cell's genome. OS markers are found in most studies of autism.
Evidence indicates a connection between autism and mitochondrial
dysfunction [53].
The dysfunction may make the ill children more sensitive to sources of
ROS, such as immune activation and pro-oxidant external toxicants.
Various substances are reported to influence the condition. Often there
is a connection to the unifying theme of ET-RSO-OS. A report describes
autism as a form of heavy metal toxicity [54].
Lead and mercury are believed to be the main causes of autism.
Detoxification by chelating agents leads to improvement in the condition
[55]. A 2008 review article deals with the mercury provoked autism in children [56]. Related articles showed severity of autism is associated with toxic metal body burden and red blood cell glutathione levels [57,58]. A study showed the connectivity between toxic metals in the hair and autism spectrum disorder in young children [59,60]. The Introduction points to the role of heavy metals in the thesis of ET-ROS-OS, Butyl paraben (Figure 2), a potent estrogen, is a preservative which is suspected as contributor to the autistic condition [61].
The compound increased OS, decreased GSH levels, elevated GSSG levels,
elevated mitochondrial dysfunction and increased protein oxidation,
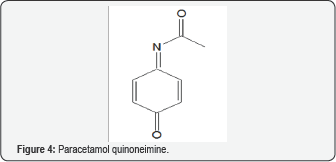
along with increase in 3-nitrotyrosine. Phenols, of which Fig. 2 is a part, can exhibit both oxidant and AO properties [24]. Paracetamol (Figure 3) has been implicated with autism in various reports [62]. OS may be involved in the mechanism, possibly by means of the quinoneimine metabolite (Figure 4) [9].
The AO vitamin D appears to play a role. Supplementation increased
total plasma AO capacity. Activities of SOD, GSH reductase and GSH
peroxidase are associated with the serum vitamin. Levels of the vitamin
are lower with the affected children. Precoporphyrin, a specific
indicator of metal toxicity, was also elevated in autistic disorder.
Treatment with dimercaptosuccinic acid, an AO, led to a significant drop
in urinary porphyrin excretion [63]. Other insults that enhance OS are involved, such as toxins, fever, infection and inflammation.
An ecological study provides evidence for involvement of environmental factors [64].
CNS pathology, including OS, neuro inflammation and mitochondrial
dysfunction, suggests involvement of environmental factors, e.g.,
pollution [65].
Biochemical abnormalities, such as GSH imbalance, may play a role. A
report indicates that autism and Parkinson's disease display commonality
in OS from toxins [66].
There may be difficulty in handling ROS. AO therapy may be beneficial.
Developmental neuro toxicants are shown to play a role in autism [67].
Examples are manganese (Mn) fluoride, chlorpyrifos and brominated
diphenyl ethers. Genetic factors appear to account for less than 30-40%
of neuro developmental disorders.
Therapy with dimercapto succinic acid is fairly
effective and safe in counteracting the toxicity of several toxic
metals, particularly lead [68]. There is evidence that hyperbaric oxygen can be beneficial in autism [69].
Data reveal that OS is reduced by this treatment through the up
regulation of AO enzymes. Also, there is increase in the formation of
mitochondrial enzymes that assist in detoxification. A report describes
autism as an epidemic and advances the hypothesis that toxicity and OS
may contribute to neuronal insult [70]. There is possible involvement of GSH, a common in vivo AO which combats unwanted OS.
Reviews puts focus on the many ways that OS may play a role in autism [44,71].
Lipid per oxidation is involved with increase in OS. Increased
inflammation, excitotoxicity, as well as mitochondrial and immune
dysfunction, appear to play a role. There are various reports dealing
with the favorable effects of AOs in reversing OS. Concentrations of
major AO serum proteins are decreased along with alterations in
activities of AO enzymes, such as GSH and homocysteine. A study revealed
changes in nitric oxide levels and antioxidant enzyme activities may
have a role in the patho physiological mechanisms involved in autism [26,72].
A mechanism links OS with membrane lipid alteration, inflammation,
altered immune response, excitotoxicity and impaired energy metabolism.
Isoprostanes, which is a product of lipid per oxidation, is enhanced in
autism [73]. In addition to OS, endothelial activation could play a role in autism.
Genetics and associated factors
Evidence indicates that about 25% of autistic children suffer from genetic influences [74].
The review identified the genes involved in autism and addressed causal
theories. Autism is believed to be one of the most heritable of mental
disorders [75].
Studies, which have been faulted, estimate the figure to be more than
90%. Various neuro psychotic disorders appear to posses common genetics.
A review describes autism as a neurological disorder with pronounced
genetic input, but with appreciable environmental involvement [76].
Evidence indicated that rates of the illness were markedly associated
with congenital malformations of the male reproductive system [77]. A study addresses the role of co morbidity in autism [78].
The underlying illness mechanism is probably polygenic and perhaps
epistatic with interaction of genetics and environmental factors [79]. A report suggests that autism is not a single disorder, but has multiple characters [80]. Brain developmental syndrome may play a role.
Autism is believed to be the most heritable of neuro developmental disorders [81].
A study with twin pairs showed moderate genetic heritability together
with a substantial shared environmental component. A conclusion is that
genetic factors involved with autism susceptibility have been
overestimated. An article is titled "Searching for ways out of the
autism maze" [82].
The ways discussed include genetic, epigenetic and environmental
involving complex patho-genetic pathways. A related report addresses
both genetic and environmental factors [83].
Animal models are described that occur following insertion of different
autism related genes. Both genes and the environment can alter the
structure of the developing brain in different ways. A research study
deals with common genetic variants, acting additively, as a major factor
for autism risk [84].
A myriad of genetic variants of small effects input autism liability.
There is an overview of genetics, including causes, therapy, and
treatment [85].
Heritability is believed to comprise at least 80% for autism, bipolar
disorders and schizophrenia, similar to diabetes, but more than for
breast cancer or Parkinson's disease [86]. A review deals with phenotypes of autistic disorders within the group of autism spectrum disorders [87].
Emphasis is on whole genome screens. Another review discussed the
contribution of epigenetic to the understanding of genetic factors in
autism [88].
Epigenetic refers to changes that alter expression of genes without
changing DNA sequence, and considers the role of environmental
contributions. Evidence is presented for epigenetic dysregulation in
autism. An investigation deals with familial risks of autism [89].
In Sweden, the risk of autism increases with increasing genetic
relatedness. Heritability of the disorder appears to be about 50%. A
2014 article suggests from a large study in Sweden that about half of
the risk for autism comes from genetics and the other half from
environmental factors [90].
Spontaneous mutations appear to play an important role in autism risk. A
mathematical model in California attributed 38% of risk for autism to
genetic and 58% to the environment. Another analysis suggested that up
to 90% of autism is genetic rather than environmental. Underlying
mechanisms are addressed concerning genetics in association with
environmental factors [91].
The role of epigenetic mechanisms is discussed. A redox/ methylation
hypothesis was advanced to rationalize the cause of autism based on the
combination of genetic and environmental factors [92].
Autistic children exhibit evidence of OS, including a relation to
methylation. A unique membrane signaling process is impaired in the
condition. Genetic polymorphism occurs more frequently in autistic
children. OS, initiated by environmental factors, leads to neurological
deficits. A 2015 investigation finds that genetic aspects provide
substantial impact in etiology of autism [93].
Vaccine
There has been recent attention concerning autism and vaccine [94,95].
In 1998, a report suggested a link between autism and vaccine.
Vaccination rates dropped because parents were concerned about a
possible connection between autism and vaccine. Soon thereafter studies
were reported refuting the link [96].
Cell signaling
Various excitations can complicate pathological problems by excitotoxicity and microglial priming [97].
Also, there are effects on cell signaling that can influence
neurodevelopment and neuronal function. We suggest that ET can play a
role, as for metal toxins. A review presents molecular processes that
have been implicated in the illness [98]. Among the various factors is cell signaling. Cell adhesion molecule (CAM) pathway genes are associated with autism [99].
The CAM pathway is important for normal cell signaling. Biological
components are part of an integrated network that permeates all aspects
from gene regulation to cell signaling and neuronal activity [100].
The concept has been applied to various illnesses including autism. An
epidemiologic investigation was performed on genetic and environmental
factors concerned with autism [101].
Cell signaling was among the various contributing items. The expression
abnormalities of genes in autism were evaluated and their roles in cell
signaling events are addressed [102].
Other factors
The effect of fetal stem cell transplantation on autism was investigated [103].
No adverse effects were found in the treated children. Statistically
significant favorable differences were noted. The results may be of
therapeutic value. Important brain function in human can be attributed
to polyunsaturated fatty acids of which docosohexaenoic acid (DHA) has
particular importance [104].
Many developmental disorders, such as autism, are causally related to
lower levels of DHA. We believe that extended conjugation in DHA may
play a part in ET and ROS involvement.
Acknowledgment
Editorial assistance by Thelma Chavez is acknowledged, as well as literature searchers by Darlene Nowak and Linda Muroi.


Comments
Post a Comment