Preparation and Application of Novel Chitosan-Guar Gum Composite as Drug Delivery Carrier-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
The aim of present work was to prepare the composites of ionic polymer chitosan with non ionic polymer guar gum and then to evaluate it as an excipient for the fast disintegrating drug delivery systems. Chitosan gel (5%) was prepared using acetic acid solution (2%) while 5% guar gum solution was prepared using deionised water. Composite was prepared by mixing both polymers in same proportion at regulated temperature. The dried and sieved composite was characterized for its micromeritic properties, flow behaviour, swelling behaviour and surface characteristics. Six batches of tablets Were prepared with composites in different ratios viz. 5, 10, 20, 30, 40 and 50% of total tablet weight, using phenytoin sodium as model drug. These tablets were evaluated for hardness, friability, weight variation, in-vitro disintegration time, in-vitro release characteristics and stability study using official methods. All the physical parameters were found within the standard limits. Batch F2 showed best in-vitro release and released about 90% drug within first five minutes. Results revealed the fact that characteristics of all tablets did not change significantly for duration of six months. Thus it can be concluded on the basis of present study that these composites can act as excellent excipient for the fast disintegrating drug delivery systems in chronic disorders.
Figure 1: Scanning electron microscopic (SEM) photograph of the surface of chitosan-guar gum composit

Keywords: Composite; Biocompatible polymer; Fast disintegrating tablets; Stability study; phenytoin sodium
Introduction
Composites are generally formed by physical interaction between two different polymer solutions. This type of composites exhibit unique physical and chemical properties, as the interactions within the polymer gels have considerable effect. Many studies have been done to use composites of chitosan and anions for pharmaceutical applications [14]. Chitosan, poly-b-(1-4)-2-amino-2-deoxy-D-glucose, is a naturally occurring cationic polysaccharide derived from the N-deacetylation of chitin. At acidic pH ranges, the ionizable amino groups in chitosan molecules are protonated [5,6]. The formation and the properties of composite depend on various factors including nature and position of the ionic groups, charge density and concentration of both polymers; it also depends upon proportion of charges, molecular weight of the macromolecules and condition of synthesis [7-10]. Most of the natural polysaccharides are anionic in nature such as gum acacia, alginate, xanthan gum, guar gum and gellan gums etc. Guar gum is a naturally occurring polysaccharide obtained from the endosperm of Cyamopsis tetragonalobus. Structurally it is a galactomannan having galactose to mannose ratio of approximately 1:2 [11]. Guar gum is obtained from natural sources and thus a renewable resource, biodegradable and non-toxic. We expect some improvement in the properties (such as hydrophilicity) to be solely based on interaction between two different natural polymers. This paper attempts to investigate the feasibility of chitosan-guar gum composite, as excipient for fast disintegrating drug delivery system in treatment of chronic disorders using phenytoin sodium as model drug. Phenytoin sodium is commonly used anticonvulsant which is useful in the treatment of status epilepticus of the grandmal seizures type. Tablets prepared using chitosan-guar gum composite expect to show good mechanical properties in terms of better hardness and less friability as compared to single polymer used. Hence this formulation overcomes transportation and handling problems generally found in commercial fast disintegrating tablets. This type of polymer promotes oral delivery of drug within few seconds, thus better patient compliance can be achieved for unconscious patient.
Materials and Methods
Material
Drug Phenytoin sodium was purchased from Alchem Laboratories, Baddi India. Sodium alginate and microcrystalline cellulose were procured from CDH Laboratory reagent, Central Drug House (P) Ltd, New Delhi, India. Chitosan (Medium molecular weight, viscosity 200.000cps) was purchased from Sigma Aldrich, Spruce Street, St. Louis. All other materials were of pharmaceutical grade and purchased as "supplied without further purification".
Preparation of chitosan-guar gum composites
5% chitosan gel was prepared using 2% acetic acid solution. Continuous agitation and heating made a gel formation at temperature about 45 °C. Further temperature was increased up to 70 °C with continuous stirring. Guar gum was added in deionized water to form homogeneous solution of 5%(w/v). Temperature of gum solution was increased slowly up to 60 °C. To prepare composites, equal proportion of both solutions was mixed with continuous stirring and temperature of mixture was reduced within the range of room temperature (25-35 °C). This solution was further dried under vacuum. Dried composite was powdered and passed through sieve #20, and stored in airtight container for further study [12].
Experimental work
Characterization of chitosan-guar gum composites
Surface morphology of composite particles: The surface characteristics of composites were observed by scanning electron microscopy ((Leo 435 VP, Carl Zeiss NTS GmbH, Oberkochen, Germany). Dried powdered composites were passed through sieve #20 and coated with gold using a sputter coater (Agar sputter coater, Agar Scientific, Stansted, UK) under high vacuum (100m Torr) and high voltage (1.2kV and 50mA). The samples were imaged using high energy electron beam.
Micromeritic properties of composite: As described by authors elsewhere apparent bulk density (g/ml), tapped sensity, Carr's Index, bulkiness and angle of repose of powdered composite was determined [12,13].
Swelling study: As discussed earlier swelling properties of composite was studied [14].
Rheological study of coacervates: To determine viscosity 1%w/v solution of composite was prepared in purified water. Viscosity of polymer was measured at shear rates from 0.1 to 2.0s-1 at 25 °C using Brookfield viscometer.
Preparation of composites based tablets
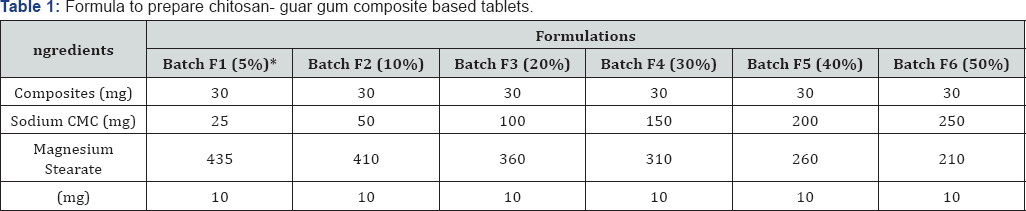
*percentage of total tablet weight
Granulation: In present research work, wet granulation technique was used to prepare matrix tablet. Drug dose (phenytoin sodium) was 30mg for each tablet and was added accordingly. According to Table 1, all the ingredients were mixed physically for 25min, using a mortar pestle. Then, appropriate amount of distilled water was added to prepare a wet mass. Wet mass was passed through sieve 20 # to obtain granules. The granules were dried at 45 °C for 24h.
Preparation of tablets: Equivalent to weight of single tablet (500mg), granules were weighted and compressed by Cadmach punching machine with 12mm diameter flat faced tolling.Tablets were compressed at compression force of 2 Newton for 10 seconds [12].
Evaluation of composites based tablets
Evaluation for weight variation: Test was carried out according to the European pharmacopoeia. Twenty tablets were randomly selected from each batch and the mean of tablet weights was calculated. Results are presented as mean value and standard deviation [12-14].
Tablet thickness testing: The thickness of the matrix tablets was determined using vernier caliper (Mitutoyo Dial Thickness Gauge, Mitutoyo, Japan) and the results were expressed as mean values of 10 determinations with standard deviations [15-17].
Evaluation for tablet hardness: Hardness of all batches was determined using Digital Force Gauge (Model:EL=500N, Electrolab). The test was carried out in triplicate for all batches as per USP XXIV monograph for uncoated tablets. The tablet hardness was expressed in Newton (N) unit and mean with standard deviation were calculated [12-14].
Friability measurement: Twenty tablets were randomly selected from each batch, accurately weighed and placed in the drum of friabilator (Erweka type, GmbH, Germany). The tablets were rotated at 25rpm for a period of 4min and then removed, dedusted and accurately re-weighed (EP 2000). The percentage loss in weight was calculated and taken as a measure of friability. The readings were shown as mean of triplicate with standard deviation [12-14].
Drug content: The tablets were powdered, and 30mg equivalent weight of phenytoin sodium in tablet powder was accurately weighted and transferred into a 100ml volumetric flask. Initially, 10ml of phosphate buffer (pH 6.6) was added and shaken for 10min. Then, the volume was made up to 100ml with buffer. Subsequently, the solution in volumetric flask was filtered, and 1ml of the filtrate was diluted and analyzed at 252nm using UV-visible spectrophotometer (Shimadzu UV-2450, Japan). The drug content of the each sample was estimated from standard curve [12-14].
In-vitro disintegration time: Tablets of all batches were selected and evaluated for disintegration time in distilled water maintained at 37±0.5 °C temperature using a disintegration apparatus (Electrolab, TDT-06T, Mumbai, India ), according to European pharmacopoeia (2002) specifications. The disintegration time was defined as the time necessary for the "Oral Disintegrating Tablet" to completely disintegrate until no solid residue remains or only a trace amount of soft residue remains on the screen. A digital stopwatch was used to measure the disintegration time to the nearest second. Only one tablet was analyzed at a time in order to ensure accuracy. All results are presented as mean value of six readings with standard deviation [12-15].
In vitro drug release study: In vitro drug release was studied using LabIndia Dissolution apparatus, with 900ml of dissolution medium (phosphate buffer pH 6.6) maintained at 37±1 °C for 90min, at 100rpm. 5ml of sample was withdrawn at particular time interval, and was replaced by an equal volume of fresh dissolution media of same pH (phosphate buffer pH 6.6). Collected samples were analyzed spectrophotometrically at measured wavelength of 252nm, and cumulative percent drug release was calculated. The data obtained in the in-vitro dissolution study was analyzed in terms of, percentage drug release with respect to time (min).
Kinetic assessment of in vitro drug release: To determine the drug release pattern based on release model, the in vitro drug release data were fitted to Zero-order (cumulative percentage amount of drug release versus time), First-order (log cumulative percentage of drug remaining to release versus time), Higuchi (fraction of drug release, Mt/Mi, versus square root of time) and Korsemeyer-Peppas (log fraction of drug released, log Mt/Mi, versus log time) models. Most suited model for drug release was decided on the basis of regression coefficient.
Stability study: Selected tablets of all formulations were stored in polyvinyl chloride (PVC) blisters covered with aluminum foil, at room temperature and 60% relative humidity for a time period of 12 months. To maintain relative humidity, ammonium nitrate (NH4NO3) saturated salt solution was used as a humidifier. Stability of all formulations was assessed by comparing the results from in vitro disintegration and dissolution studies. To investigate any changes in the physicochemical properties of composite and drug, infrared spectrum of individual compound was matched after 0, 3, 6 and 12 months interval. The results were checked for statistical significance using the one-way analysis of variance (ANOVA), F-test for testing the equality of several means. A p-value>0.05 was considered statistically insignificant [16].
Results
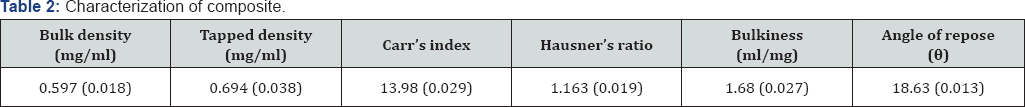
#value in parenthesis show standard deviation of triplicate readings.
Chitosan-guar gum composite was synthesized and characterized for micromeritic properties and flow behaviour; findings of these studies are shown in Table 2. Scanning electron microscopic study of composites easily depict the fact that surface of composite particles were rough in nature. As per the table 3, the formulated matrix tablets met the Pharmacopoeial requirement of uniformity of weight. They also confirmed, to the requirement of assay, as per USP. Hardness, percentage friability and thickness were all within acceptable limits. The hardness of tablets was determined and was found to be in the range of 20.08 to 20.88 N. The values for all batches showed that tablets have sufficient hardness and further friability was studied for all batches. Friability was obtained between 0.112 to 0.212%, which was below 1% indicating sufficient mechanical integrity and strength of the prepared tablets. Thickness of tablets was also measured to evaluate efficacy of process of formulation development. Thickness of tablet was found in between 3.74 to 4.10mm. Drug content was also measured to evaluate the accuracy of procedure. Good and uniform drug content values for all batches are able to show that this particular process was quite efficient.
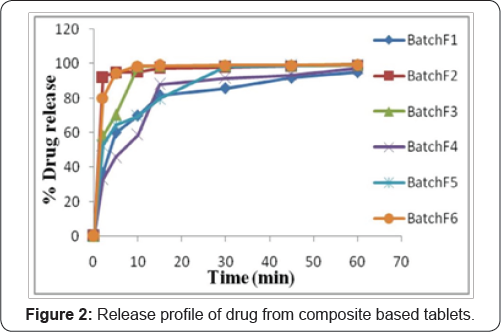
Results of in vitro release study showed that all studied tablets released more than 95% drug within 60min as shown in Figure 2. Batch F2, batch F3 and batch F6 release more than 90% drug within 15min and which is comparatively better than batch F1, batch F4 and batch F5 (Figure 2). Results obtained from in vitro drug release were fitted to Zero order, First order, Higuchi kinetics and Korsmeyer-Peppas diffusion model (Table 3). Model that best fitted the release data was evaluated on the basis of correlation coefficient (r). Findings of Table 4 easily predict that none of the formulation showed better regression value when fitted to zero order equation (Qt=Q0+K0t). When release data was plotted according to first order equation (Qt=Q0e-K1t),batch F1, F2, F4 and F5 showed linearity, with regression value 0.944, 0.929, 0.904 and 0.979 respectively. Higuchi kinetics was fairly shown by batch F5 with regression value 0.919, other batches did not show significant regression valve when fitted in Higuchi kinetic equation (Qt=Kh t1/2). Diffusion mechanism of drug release was studied by fitting in vitro drug release data in to Korsmeyer-Peppas diffusion model (Qt/Qt=Kktn). All batches except Batch F5 showed good linearity of regression coefficient with this model. On the basis of regression value of all batches it was concluded that batch F1, F5 and F6 follow First order kinetics (release rate of drug from matrix, is proportional to the amount of drug remaining in the matrix) while batch F2, F3 and F4 follow Korsmeyer-Peppas diffusion kinetics (drug release based on more than one phenomenon) with n value less than 0.5 (fickian diffusion).
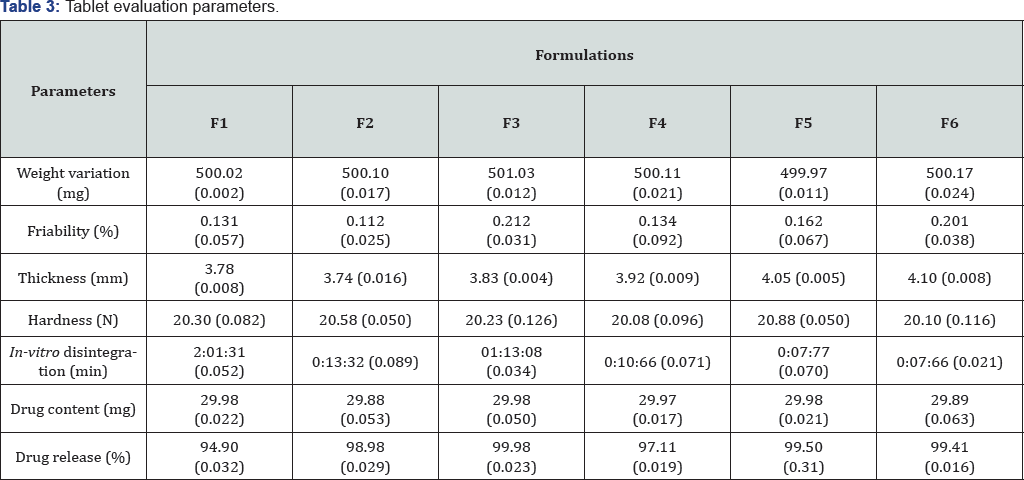
Values in parenthesis shows standard deviation.
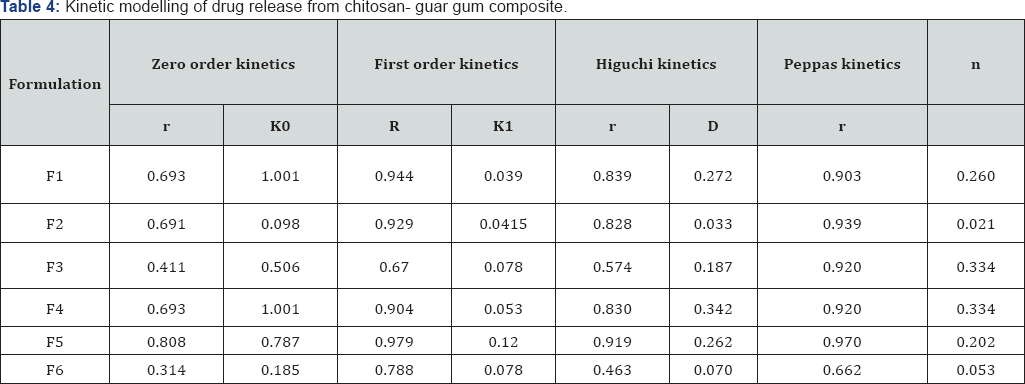
¶r=Correlation Coefficient; K0=Zero Order Release Rate Constant; K1= First Order Release Rate Constant; D=Diffusion Coefficient and n= Release Exponent
After 12 months of stability study at controlled environmental conditions no significant differences (p<0.05) in disintegration time and in vitro drug release rate of the prepared tablets were observed. On the basis of infrared spectroscopic studies during the stability study, it can be concluded that there was no change in the physico-chemical properties of both composite and drug (Table 4).
Discussion
Findings of present investigation demonstrate that the properties of composites are entirely different from the properties of each single polymer from which that complex is synthesized. Synthesized composites have characteristics properties in terms of micromeritic properties and flow behaviour (Table2) . Micromeritic study data is important in predicting the flow property of polymer as well as for granules obtained after granulation. The synthesized composites showed better flow properties hence depicting the fact that this complex when used as polymer would enhance the flow characteristic of the formed granules. Flow property is emphasized because, it decides the ease of compaction of powder or granules into a tablet dosage form. Carr's index was studied with the aim to evaluate compressibility characteristics of the composite. All these parameters ranged between the reported limits. Results also reveal the fact (Table 3)that micromeritic properties and flow characteristics does not changed significantly when different powder blends were prepared using composite. Post compression study such as hardness and friability were all within acceptable limits. These are important physical properties which demonstrate that the tablet formulated can be handled easily without any physical damage. These two data show the importance of formulation over commercial fast disintegrating tablets which have problem during transportation and storage. Thickness of tablets was also measured to evaluate efficacy of process of formulation development. Almost uniform thickness of tablets showed that this process is efficient enough to produce tablet. Drug content was measured to evaluate the efficiency. Uniform drug content values for all batches show that this particular process was highly efficient and can be used at industrial level to formulate fast disintegrating tablet for a particular disease condition. The in vitro drug release was studied in terms of % drug release vs time (min) graph, and showed that all batches released drug within a small duration of time. Hence, release characteristics are able to demonstrate that all the formulations can be used for the commercialization of product. The drug release data for the various chitosan-guar gum composite tablets fitted into the classical power law expression. This indicates that drug release from these matrix tablets followed non-Fickian kinetics, due probably to rapid swelling and erosion of the matrix structure formed by the interaction of chitosan and guar gum. It is likely that the incorporation of composite into tablets would enhance initial swelling, followed by erosion of matrix. Data obtained during stability study demonstrate that these tablets were stable during the period of stability study (Table 4). Properties of tablets did not change significantly during the study. So these types of tablets can be stored in the environmental condition (temperature-25 °C, relative humidity 40% to 60%).
It can be concluded by all the physico-chemical parameters and in vitro disintegration and in vitro dissolution study that batch F2 was optimized formulation among the six batches of tablets. The batch F2 showed less friability and greater hardness when compared to other batches. In vitro disintegration time and in vitro dissolution time were also less comparatively. The tablets released 98.98% of drug. It can be concluded on behalf of obtained results that neither the drug nor the polymers show any interaction, thus removing the uncertainty of cause of any chemical interaction. This is utmost important while formulating any dosage form, so that individual characteristics of compound is retained Table 5.
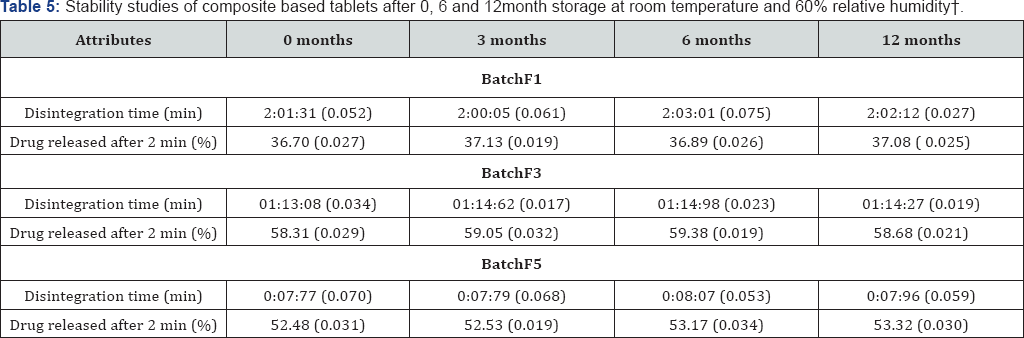
┼valves in parenthesis shows standard deviation of triplicate readings
Conclusion
The study demonstrated that physical interaction took place during the formation of chitosan-guar gum composites which helped in enhancing the release of drug from the tablets within few minutes. Thus, this may prove to be efficient means of formulating fast disintegrating tablets for the treatment of chronic disorders at the initial stage of attack/onset.
Acknowledgement
Authors are highly thankful to "Sophisticated Analytical Instrumentation Facility (DST)" Department of Anatomy, All India Institute of Medical Sciences, New Delhi India, to carry out scanning electron microscopic (SEM) study of chitosan-guar gum composite.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/


Comments
Post a Comment