Synthesis and Characterization of New Triazinane-2-Thione and Oxadiazinane 4-Thiones from Benzimidazole -Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
The present study a new strategy for the synthesis of most potent nitrogen heterocycles i.e., triazinane-2-thiones and oxadiazinanes-4- thiones (3-4) were efficiently synthesized by cyclic condensation based on classical Mannich amino methylation of N, N' unsymmetrical Thio ureas. The synthesized compounds have been characterized by analytical and spectral (IR, 1HNMR and Mass) data.
Keywords: Synthesis; Characterization; Triazinane-2-thione; Benzimidazole
Introduction
Demand for higher efficiency, economy and selectivity in the synthesis of novel molecular scaffolds drives organic chemistry, particularly nitrogen-containing molecules has been of longstanding interest to organic chemists due to their great importance in chemistry and biology [1]. Many of these heterocyclic compounds exhibit various biological activities [2,5]. Little work has been published in the area of 1,3,5 triazinane- 2-phones showing antimicrobial activity [6], Biocidal [7] and enantio-differentiating coupling reagents [8]. The potent Heterocycles having, structural unit has a significant place among pharmaceutically important synthetic and natural materials [9].
Materials and Methods
Melting points of the synthesized compounds are determined in open capillary tubes and are uncorrected. Reaction Progress was observed by TLC plates, Bruker 300MHz instrument was used to record 1HNMR spectra in DMSO/CDCl3 using TMS as internal standard. Chemical shifts (5) are expressed in ppm. Perkin Elmer BX series FT-IR was to record IR spectra, Elemental analysis were performed on a PerkinElmer 240CHN analyzer Figure 1.
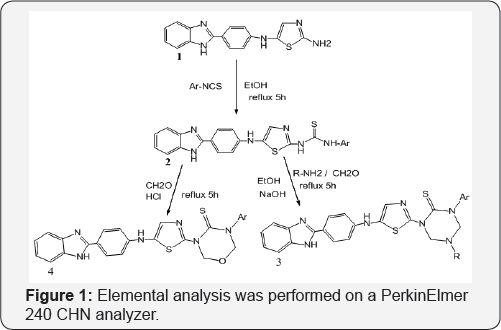
Experimental section
Synthesis of 1-(5-(4-(1H-benzo[d]imidazol-2-yl)phenyl) amino)thiazol-2-yl)-3-phenyl thiourea (2): A mixture of compound [10] (1) (0.01mmol) and sodium hydride (0.5g, 20mmol) in Ethanol (80ml) was heated under reflux for 30min and cooled. Phenyl isothiocynate (0.01mmol) was added and refluxing continued for a further 4h. The solvent was evaporated off and the residue dissolved in DCM (50ml) and washed with dilute HCl. The organic phase was dried (MgSO4) and the solvent evaporated off to give the desired compound (2). The progress of the reaction was monitored by TLC and recrystallized from ethanol.
Synthesis of 1-(5-(4-(1H-benzo[d]imidazol-2-yl) Phenylamino)thiazol-2-yl)-5-methyl-3-phenyl-1,3,5 triazinane-2-thione (3): A mixture of compound (2) in (1mmol), 30% formaldehyde (2mmol) and methyl amine (1mmol) and (0.01mole) NaOH were taken in ethanol (30ml) and refluxed for about 5-6h. The progress of the reaction was monitored by TLC. After completion of the reaction it was cooled and the product was filtered. The crude product was passed through silica gel by column and the product was eluted from 60% ethyl acetate and hexane.
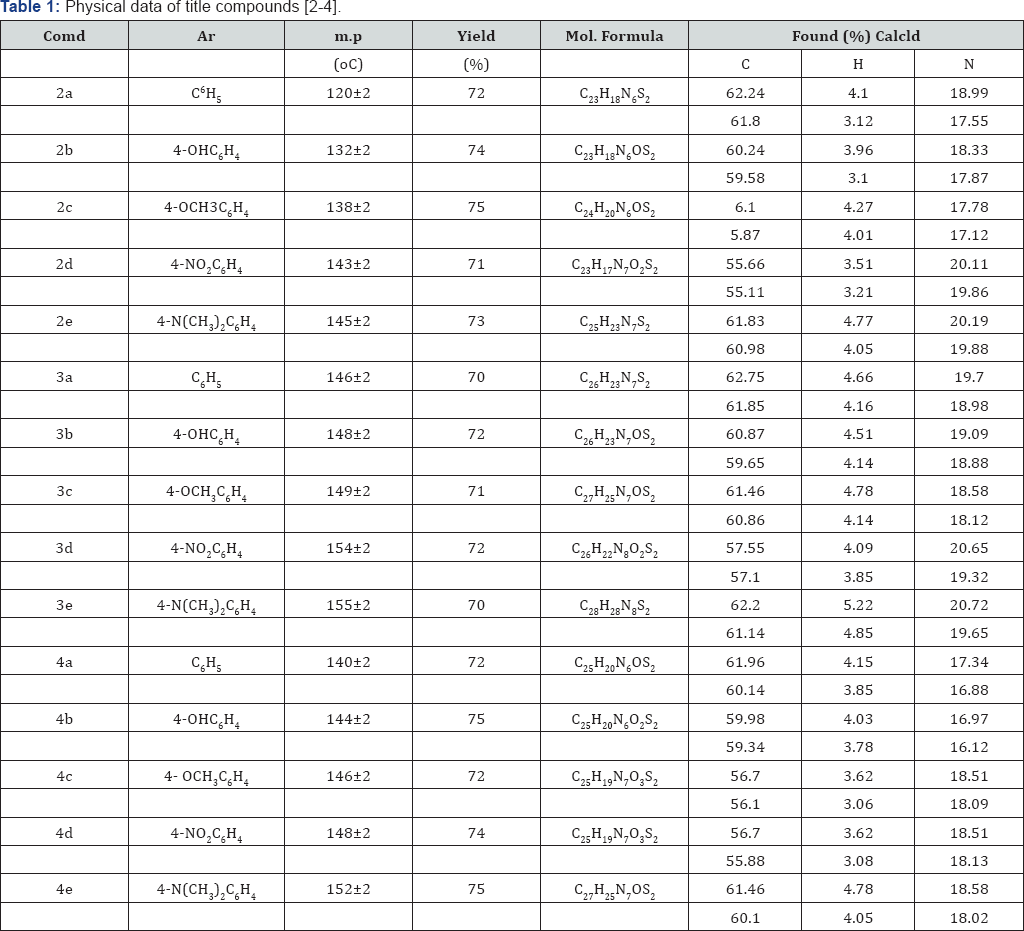
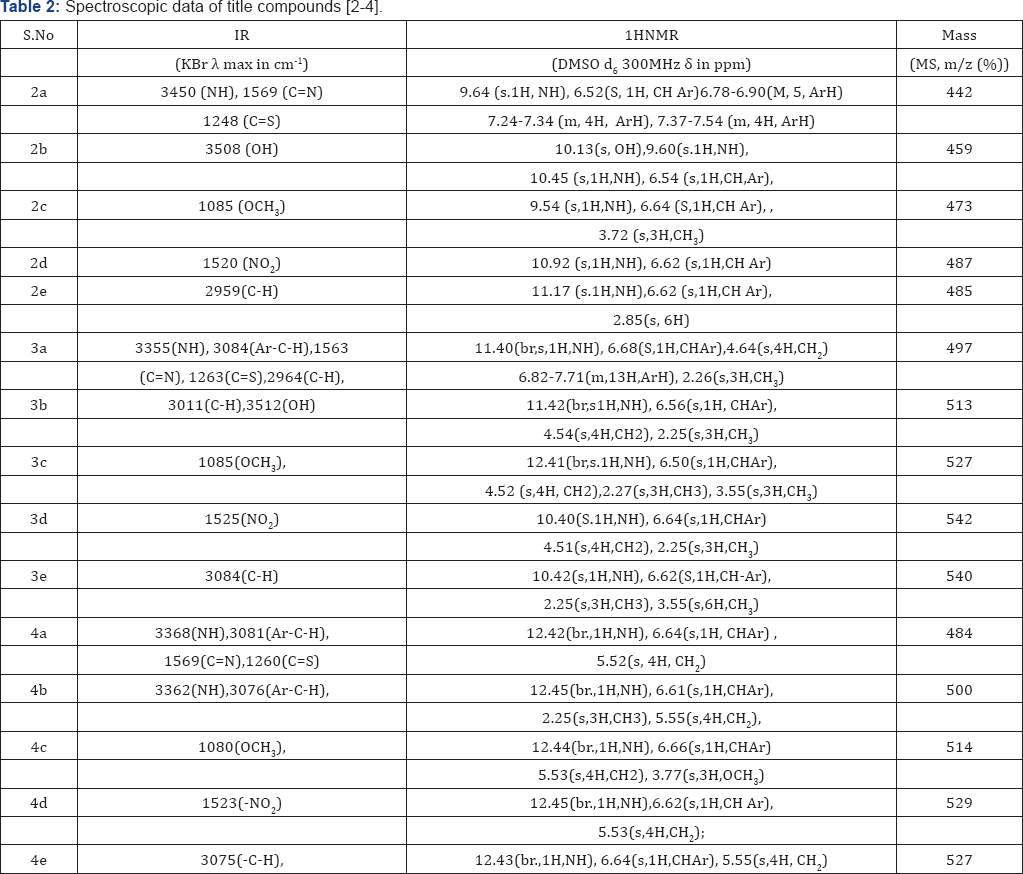
Synthesis of 3-(5-((4-(1H-benzo[d]imidazol-2-yl) phenyl)amino)thiazol-2-yl)-5-phenyl-1,3,5-oxadiazmane- 4-thione(4): A mixture of compound (2) in (1mmol), 30% formaldehyde (2mmol) were taken in ethanol (30ml) and add 1ml concentrated HCl (5ml) and refluxed for about 5-6hr at 100110 °C. The progress of the reaction was monitored by TLC. After completion of the reaction it was cooled and neutralized with 10% NaOH. The product was filtered. The crude product was passed through silica gel by column and the product was eluted from 60% ethyl acetate and hexane. These compounds were purified by recrystallisation from suitable solvents. Structures of the synthesized compounds [2-4(a-e] have been confirmed on the basis of analytical and spectral (IR, 1HNMR and Mass) data (Table 1).
Results and Discussion
In the present study, it has been discussed that the synthesis of various benzimidazole fused triazinane 2-thione heterocyclic moieties by cyclic condensation based on classical Mannich amino methylation of N,N' unsymmetrical this ureas (2) with 30% HCHO and methyl amine in ethanol. An aliphatic amine has been yielded in the condensation between methyl amine and formaldehyde, it cyclizes immediately to produce corresponding 1,3,5-triazinane since imine is unstable. With respect to biological activity benzimidazole fused heterocyclic compounds such as triazinane derivatives are of very important than the cyclic compounds [11,12] (Table 2).
Conclusion
The research study reported efficiently synthesized new analogs of triazinane and oxadiazinane by classical amino methylation with different aerial-N,N' unsymmetrical thioureas by taking benzimidazole as starting material. All the compounds were characterized by standard spectroscopic techniques.
Acknowledgement
The authors are grateful acknowledge to the Department of chemistry Kakatiya university, Warangal for their constant support during this research work.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment