In-vitro and In-silico Drug-Food Interaction: an Evaluation of Metformin and Green Tea Interactions-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
Background and Objectives: Food-drug interaction is a consequence of physical, chemical or physiological relationship between a drug and food. Failure to identify and properly manage food-drug interaction can lead to serious consequences such as reduction in absorption of certain orally administered drugs thereby leading to failure of treatments. This study sort to explore the effect of green tea on Metformin uses both in-vitro dissolution test and in-silico docking interactions models.
Method: Dissolution test was carried out on Metformin alone and Metformin in the presence of green tea using the official dissolution medium, phosphate buffer pH 6.8 and sampling done at USP timing intervals. Docking studies was carried out by using 10 phenolic compounds and metformin in the active site of the AMPK crystal structure, 4ZHX.pdb.
Results: Metformin alone complied with the USP requirement of 70% drug release while Metformin release in the presence of green tea was less than 70% at 45minutes. Phenolic constituents of green tea; (-)-epigallocatechine, epicatechine, theanine and theophylline were seen to form complexes with metformin through covalent bonding in the active site of AMPK.
Conclusion: This study was able to establish the interaction of green tea on metformin dissolution profile and possible binding interactions in the binding site of AMPK enzyme. It was therefore concluded that green tea decreases the release and uptake of metformin by forming complexes through covalent and hydrogen bounding of some of it’s phenolic constituents.
Keywords:
i. The presence of green tea in the dissolution media along with metformin caused a decrease in its dissolution profile due to complex formation
ii. The catechins and theanine constituents of green tea could possibly compete for binding site residues with metformin.
Abbrivations: CAMP: Cyclic Adenosine Monophosphate; PKA: Protein Kinase A; SPSS: Statistical Package for Social Sciences; AMPK: AMP- activated protein kinase
Introduction
Food has been reported to alter pharmacokinetics and effect of concomitantly administered drugs, hence resulting in a food-drug interaction [1]. Food-drug interaction is defined an alteration of the pharmacokinetics and pharmaco dynamics of a drug by food or food ingredients. Failure to identify and properly manage food-drug interaction can lead to serious consequences such as reduction in absorption of certain orally administered drugs thereby leading to failure of treatments. Induction and inhibition of enzymes in the gut by food ingredients may lead to a significant change in bioavailability of drugs by some food ingredients. Adverse effects of some drugs can be potentiated by food ingredients [2]. Green tea is one of the foods that have been reported to cause food drug interaction with metformin [2]. Metformin (dimethyl biguanide) is an orally administered drug used to for glycemic control in patients with type II diabetics [3,4]. Metformin according to national guideline is recommended as first-line treatment due to several factors which includes safety record, reduction in cardiovascular disease and mortality compared with non-intensive treatment [5].
Under fasting conditions, the bioavailability of metformin is 50-60%. Metformin has slow absorption and a negligible plasma protein binding and reaches a steady state in one or two days. The immediate- release metformin reaches peak plasma concentration (Cmax) within one to three hours after administration while the extended-release formulation is four to eight hours. Metformin exist largely as hydrophilic cationic species at physiological pH values with the acid dissociation constant value (pKa) of 2.8 and 11.5, which makes it a stronger base than most other basic drugs and less than 0.1% non-ionized form in the blood. Metformin doesn't undergo metabolism but is cleared from the body by tubular secretion and excreted in its unchanged form in the urine; metformin is undetectable in blood plasma within 24 hours of a single oral dose [6].
Potential molecular mechanism of action of metformin has been proposed which includes inhibition of the mitochondrial respiratory chain (complex I), activation of AMP-activated protein kinase (AMPK) an enzyme that plays an important role in insulin signaling, whole body energy balance and the metabolism of glucose and fats , inhibition of glucagon-induced elevation of cyclic adenosine monophosphate (CAMP) with reduced activation of protein kinase A (PKA), inhibition of mitochondrial glycerophosphate dehydrogenase and an effect on gut microbiota [5].
Green tea (Camellia Sinensis) has positive health effects and widely consumed beverage [1,7,8]. Green tea contains an array of components amongst which are xanthine derivatives (Caffeine, Theophylline and Theobromine), Glutamide derivative (Theanine) and Phenolic compounds known as Catechins (Catechin, epicatechin, epicatechin-3-gallate, epigallocatechin and epigallocatechin-3-gallate) [9,10]. Catechins makeup 95% of green tea phenolic content and as such would be involved in a lot of green tea's interactions and activities [11,12].
Sodium hydroxide pellets (Kermel, china), potassium dihydrogen orthophosphate (Kermel, China) were obtained from the Department of Medicinal and Pharmaceutical Chemistry, University of Jos. Shimadzu UV. 2650PC Double beam spectrophotometer (Shimadzu, 1620 Japan) and RC-6 Dissolution test apparatus 1L per vessel (Tianjin China) of the University of Jos. Two brands of Metformin hydrochloride 500mg tablets were purchased from a pharmacy outfit in Jos Table 1.
Materials and Methods
One teabag was placed in a 500ml beaker and 250ml boiling water was added and stirred. This was allowed to stand for 10min before the tea bag was taken out.
Dissolution test procedure
The official USP (2007) Pharmacopoeia for dissolution testing was adopted. Using phosphate buffer pH 6.8 at temperature of 37 °C±5 °C at 100rpm the tablets were placed in the basket and the machine was adjusted to start counting down from 60 minutes. As the procedure commenced 5mls of the dissolution sample was withdrawn at 5, 10, 15, 30, 45 and 60 minutes and immediately replaced with 5mls of phosphate buffer for control and phosphate buffer with green tea for the test, to maintain sink condition.
The samples were filtered through 0.45|im syringe. The filtrate was diluted using 1 in 20 dilutions using phosphate buffer for the control and phosphate buffer together with green tea for the test. These samples were analysed by ultraviolet spectrophotometer at 232nm [13,14].
The percentage drug released was calculated using the formula:
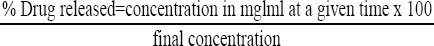
The percentage drug released was plotted against time.
1st phase (normal dissolution testing procedure) involved the use of buffer solution and two brands of Metformin tablet 500mg (control media).
2nd phase involved combination of buffer and green tea, in the ratio of 2:1 as the medium.
3rd Phase involved the spiking of the solution from 1st phase with graded amount of tea solutions just at the point of taking absorbance.
Docking studies (Computer docking process)
All methods used in the docking study were entirely Insilco, making use of several web interfaces, online databanks and software applications.
a. Data banks Protein databank (www.rcsb.org) [15]: a database providing information of proteins was used to obtain the x-ray structure of AMPK, 4ZHX.pdb [16].
b. Software applications BIOVIA Discovery Studio: a programme to examine structure properties of both large and small molecules [17]. Argus Lab 4.0.1: software for molecular modelling and drug designing [18].
Library design of ligands: A set of compound library of 10 polyphenolic constituent of green tea and metformin structures were drawn up in BIOVIA Discovery studio visualizer and Molecular Mechanics geometry optimisation performed on them. Time (Minutes)
Preparation of molecular target: The crystal structure of AMPK enzyme with the pdb code 4ZHX. Pdb was downloaded from the protein databank (http://pdb.org). The pdb file was opened in WordPad, the water molecules were deleted and the file resaved. The valance and hybridisation of the AMPK structure was checked in Argus Lab. The structure was optimised by locking all atoms except the hydrogens and Molecular Mechanics geometry (MM2) performed on it. The active site in AMPK structure was defined by selecting residues in the hydrophobic pocket of the E chain and grouping them as the binding site.
Virtual screening: The library of molecules was docked into the active site of AMPK using Arguslab (spread sheet style batch dockings). A fast Dock setting of flexible ligands in flexible active site- conditions in a 3000 step generation using Amber Van der Waals in an active box of 15x15x15Ao was performed with a maximum of 150 best poses. A Score scoring function was used in this study. Subsequently, the library of phenolic constituents of green tea was re- docked into the binding site of AMPK which contained the metformin structure.
Binding analysis: Binding interactions of the ligands with the AMPK protein active site was analysed using Arguslab 4.0.1 and BIOVIA discovery studio softwares.
Discussion
In-vitro food - drug interaction via dissolution test
Food drug interaction is an alteration in the pharmacokinetics and pharmacodynamics of a drug by food or food ingredients [2]. A food drug interaction can be a consequence of physical, chemical or physiological relationship between a drug and food.
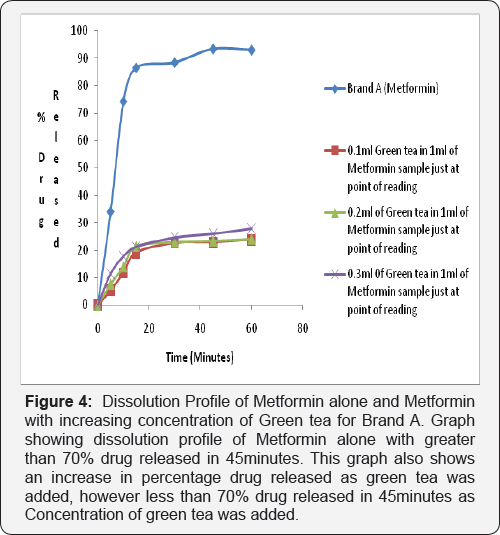
Dissolution test profile provides insight into in-vivo drug release in order to establish possible bioavailability characteristics. The USP (2007) specifies at least 70% release of drug at 45 minutes in dissolution medium for oral tablet using phosphate buffer pH 6.8. The two brands of metformin used in this study showed a percentage release of 93.41% for Brand A and 93.04% for Brand B therefore complying with the USP specification (Figure 2-4).
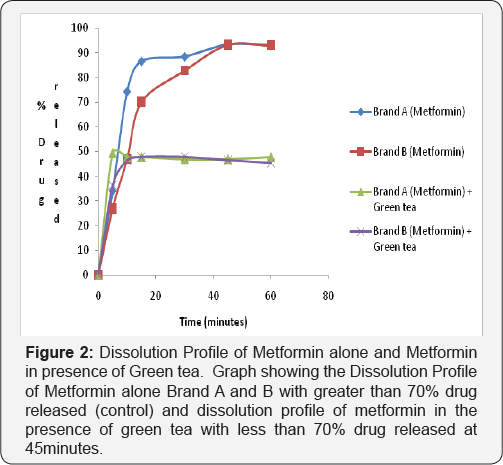
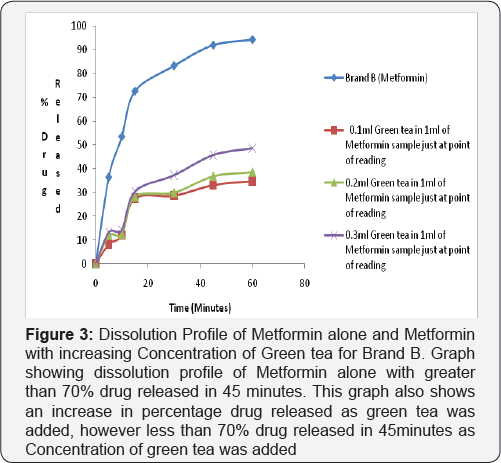
2nd and 3rd Phase: In the test sample (containing Metformin and Green Tea) there was decrease in percentage drug released of less than 70% drug released for Brand A (46.86%) and Brand B (46.46%) at 45 minutes in phosphate buffer pH 6.8. This shows that green tea interferes with the dissolution profile of metformin as shown in (Figure 4).
The test procedure for the addition of various concentration of green tea at the point of reading, showed an increase in
Ligand orientations are scored in relation to intermolecular energy interaction and ranked relative to other poses and ligands in the compound library [19]. Scoring functions such as percentage drug released and absorbance as the concentration of green tea was increased. This however did not meet this USP (2007) specification showing less than 70% drug release at 45minutes for brand A and Brand B in green tea. The increase in absorbance as varying concentration of green tea was added to metformin can be due to complex formation between constituent's green tea and metformin as shown in (Figure 4 & 5). This could also imply that constituents in green tea might not entirely stop the release of metformin, but could slow it down significantly to affect treatment outcomes in the long run.
Statistical Package for Social Sciences (SPSS) version 20 was used to calculate the student t test, which showed that green tea significantly decreased the dissolution profile of metform in tablet in phosphate buffer pH 6.8 and showed a 95% confidence level with (P<0.05). It may be inferred that green tea may cause an unpredictable decrease in the dissolution profile of metform in tablet and thus have an impact on the bioavailability of Metformin. This can be said to be a form of food-drug interaction that alters the normal dissolution profile of metformin. This agrees with the study carried out by which reported green tea decreases the uptake of metformin [1].
Predicting binding affinity from docking scores
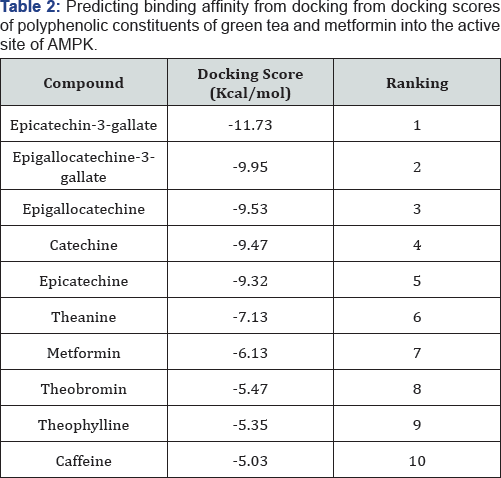
The Catechins (Epicatechin-3-gallate, Epigallocatechin, catechin, Epicatechin) and Theanine were seen to have higher binding affinity as compared to metformin in (Table 2). Theobromine, Theophylline and Caffeine were seen to have lower binding affinities than metformin.
a Score [used in this study provides a fast and predictive tool for the estimation of a ligand’s binding affinity from its binding energy. Similar docking scores between these Catechins can be attributed to having similarities in their structures. From these results, the catechins having a higher binding affinity than metformin could imply that they are competing with metformin for binding site residues.
Binding pattern analysis
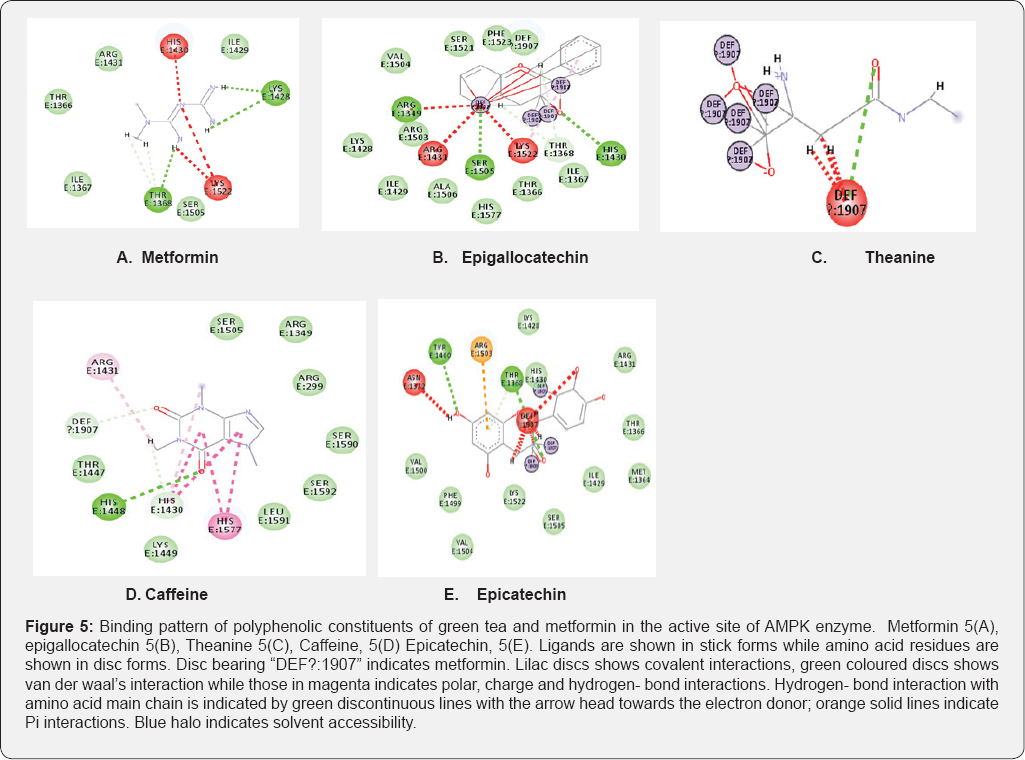
From (Figure 5a), metformin can be seen interacting with active site residues, while in the presence of green tea constituents (Figure 5b-5e), there is a shared interaction between metformin and these compounds. From this study, it was observed that interactions of Epigallocatechin, Epicatechin and Theamine with metformin (Figure 5b-5e), was through covalent bonding. The complex of green tea- metformin that would be formed through this covalent bonding interactions can explain the decrease in dissolution profile of metformin. Other interaction types such as carbon-hydrogen bonding (Figure 5d) and some unfavourable bumps (Figure 5b-5e) were also observed. These interactions can in the long run affect the bioavailability of metformin, hence interfering with treatment outcomes.
Conclusion
Green tea significantly decreases the dissolution profile of metformin tablet due to the presence of constituents in green tea which can form a complex with metformin as indicted by the in-silico dockings. Whatever affects the dissolution profile of metformin would adversely affect its bioavailability which was seen in the interaction of metformin and green tea constituents in the active site of AMPK enzyme. Regardless of the fact that some literatures showed that green tea may have health benefits with regards to decreasing blood glucose levels in diabetic patients, concurrent administration with metformin might decrease the effectiveness of the drug.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To
know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment