Formulation of Prunus Amygdalus (Family:Rosaceae) Gum Based Compression Coated Tablet for Colon Targeted Delivery of 5-Aminosalicylic Acid-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
This research deals with formulation of compression coated tablet of 5-Aminosalicylic acid and further evaluated for colon targeting ability. Drug 5-aminosalicylic acid is degraded in stomach in the presence of gastric pH so colon targeting is best way to achieve therapeutic effect. Initially core tablet of drug was prepared using microcrystalline cellulose as diluents and further it was compression coated using various concentration of Prunus amygdalus gum. Prepared tablets were evaluated for various physical and mechanical parameters such as hardness, size and shape, friability and weight variation. To evaluate drug release ability of formulated tablet in to colon, In vitro drug release study was carried out in 0.1 N HCl and phosphate buffer pH 7.4. Formulated tablets were found to be round in shape with 13.436±0.020 to 13.482±0.017mm diameter. Hardness of tablets was found to be 18.22±0.12 to 18.56±0.04. Friability of prepared tablets were within Pharmacopoeial limit (i.e. <1%). In vitro release data showed that all the prepared tablets were able to resist drug release in pH 1.2 buffer and 50% drug releases was achieved after 10h. It can be concluded from the findings of the results that Prunus amygdalus gum coated tablets were able to delivery drug 5-Amino salicylic acid successfully in to colon in sustained manner.
Keywords: Prunus amygdalus gum; Colon targeted drug delivery; 5-Aminosalicylic acid; Drug delivery; Compression coating
Introduction
In recent times, colon specific delivery systems are also gaining importance for the systemic delivery of proteins, peptides and acid sensitive drugs [1]. Due to negligible activity of peptidase and less activity of pancreatic enzymes, the colon is considered to be more suitable for delivery of these molecules in comparison to the small intestine. Besides this less hostile environment, the colonic transit time is long (20-30h) and the colonic tissue is highly responsive to the action of absorption enhancers. The longer residence time, less peptidase activity, natural absorptive characteristics and high response to absorption enhancers make the colon a promising site for the delivery of protein and peptide drugs for systemic absorption [2-4].
There are several ways in which colon specific drug delivery has been attempted1. Development of pro drugs, coating with pH-dependent polymers, design of defined release dosage forms, and the use of carriers that can be degraded exclusively by colonic bacteria are an array of such attempts. This introduction provides a review of colon function, physiology, drug candidates and various approaches for colonic drug delivery [5-8].
Prunus amygdalus exudes a gum (Almond gum), which has been employed in place of tragacanth. It is obtained mostly from the trunk. Almond gum hydrolyzes into L-arabinose (4 parts), D-xylose (2 parts), D-galactose (3 parts) and D-glucouronic acid (1 part); aldobio uronic acid is also present. This is a natural polymer and employed as coating material in present study.
5-Amino salicylic acid (5-amino-2-hydroxybenzoic acid) is an anti-inflammatory agent, structurally related to the salicylates, which is active in inflammatory bowel disease. It is considered to be the active moiety of sulphasalazine. 5-Amino salicylic acid is acid sensitive drug and degraded in low pH of stomach. Absorption site of drug is also in the colonic region. So it becomes necessary to target drug molecule in to the lower part of intestine.
Material and Methods
Drug 5-Aminosalicylic acid was received as gift sample from Cipla Ltd, Mumbai. Microcrystalline cellulose, Ethyl cellulose,Ethyl alcohol, Triethyl citrate, and magnesium stearate was purchased from Merck Ltd Mumbai and used as received. Double distilled water was used as solvent in whole study.
Preparation of core tablet

The core tablet comprising of 5-ASA and MCC was prepared by direct compression method as shown in Table 1. Accurately weighed quantities of the ingredients were mixed in a glass mortar and required quantity were placed on the KBr press and compressed at pressure of 500 Psi into tablets of 8 mm diameter.
a. Evaluation of core tablet: Prepared core tablets were evaluated for various physical parameters and mechanical properties [9-12].
b. Physical characterization: Core tablets were evaluated for following physical properties.
c. Appearance: In this the shape, color, presence or absence of an odour, taste and surface texture were recorded on five randomly selected tablets.
d. Average diameter: The diameters of five randomly selected tablets were recorded with the help of digital vernier caliper and the average diameter and standard deviation were calculated.
e. Average thickness: Thickness of the developed tablet is an important parameter to investigate. The thickness of the developed tablet was recorded using digital vernier caliper by random sampling technique. Thickness of five tablets was determined and the mean thickness and standard deviation was calculated.
Average weight and weight variation
USP procedure for uniformity of weight was followed, twenty tablets were taken randomly and their weighed was determined individually and collectively on a digital weighing balance accurately. The average weight of one tablet was determined from the collective weight. The weight variation was calculated for USP limits i.e., for average weight of 80 or less, 80-250 and more then 250; maximum percentage differences allowed are 10%, 7.5% and 5% respectively.
a. Mechanical properties: Core tablets were evaluated for following mechanical properties:
b. Hardness: The hardness of the core tablets was determined using an electrolab tablet breaking force tester USP (1217). Five core tablets were randomly selected and placed one at a time between the jaws of the hardness tester before it was
started. The force needed to break the core tablet was recorded.
c. Friability: A friability test was conducted on the core tablets using an electro lab EF-2 friabilator (USP). Ten tablets were randomly selected and any loose dust was removed with the aid of a soft brush. The tablets were weighed accurately and placed in the drum of the friabilator. The drum was rotated at 25rpm for 4 minutes after which the tablets were removed. Any loose dust was removed from the tablets as before and the tablets were weighted again.% friability was calculated using equation1.
Friability (% w/w) = (Weight before (g) - Weight after (g))/ (Weight before (g))X100 equation1
I. Drug Content of the tablet: To determine the drug content, five tablets were crushed into powder form and the powder was dissolved in 100mL of phosphate buffer, pH 7.4 and the solution was sonicated for 30min. The supernatant was filtered and the absorbance was measured after suitable dilution at 331nm.
II. Disintegration test: This test was conducted on 6 core tablets with a electro slab disintegration tester (USP) ED-2L apparatus at 37±2 °C. The phosphate buffer of pH 7.4 was used as a disintegration media. The time taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured in minutes.
III. Preparation of compression coat: The total weight of the compression coating was 300 mg for each drug: polymer ratio. The seven batches were prepared in which drug amount was kept constant and polysaccharide (almond gum) concentration varied. The ingredients were properly mixed for 5 minutes before and 5 minutes after addition of magnesium stearate (lubricant). Compression coat was prepared by wet granulation method. Accurately weighed quantities of the ingredients were mixed in a glass mortar and required quantity of distilled water was added to the powder mass and mixed thoroughly. The granules were prepared by passing the wet mass through British Standard Sieve (BSS) No. 10. Wet granules were dried in a dessicator for 36h.
IV. Preparation of compression coated tablet: 150mg of granules were placed in die of KBr press. Core tablet was placed and 150mg of granules were poured on core tablet and it was compressed in KBr press at pressure of 1500 Psi into tablets of 12mm diameter.
V. Coating of compression coated tablet: The compression coated tablet was coated with ethyl cellulose to provide delayed release action (Table 2). It is a hydrophobic polymer and thus prevent early wetting of tablet. The tablets were coated by spray coating technique. The solution of ethyl cellulose (4%w/v 20ml) was sprayed on the tablets with help of a sprayer. The tablets were dried in stream of hot air. The procedure was repeated many times until there was 50mg weight gain.

Evaluation of coated tablet: Coated tablets were evaluated for following parameters:
Physical characterization
a. Appearance: In this the shape, color, presence or absence of an odour, taste and surface texture were recorded on five randomly selected tablets.
b. Average diameter: The diameters of five randomly selected tablets were recorded with the help of digital vernier caliper and the average diameter and standard deviation were calculated.
c. Average thickness: Thickness of the developed tablet is an important parameter to investigate. The thickness of the developed tablet was recorded using digital Vernier caliper. Thickness of five tablets was determined and the mean thickness and standard deviation was calculated.
d. Average weight and weight variation: USP procedure for uniformity of weight was followed, twenty tablets were taken randomly and their weighed was determined individually and collectively on a digital weighing balance accurately. The average weight of one tablet was determined from the collective weight. The weight variation was calculated for USP limits i.e., for average weight of 80 or less, 80-250 and more then 250; maximum percentage differences allowed are 10%, 7.5% and 5% respectively.
Mechanical properties
Hardness: The hardness of the coated tablets was determined using an electro lab tablet breaking force tester USP (1217). Five coated tablets were randomly selected and placed one at a time between the jaws of the hardness tester before it was started. The force needed to break the coated tablet was recorded.
In vitro Release Behavior:
Experimental Conditions:
Apparatus - USP Dissolution Apparatus, Type II (Paddle)
Dissolution Medium- SGF pH 1.2, Phosphate buffer pH 6.8 and Phosphate buffer pH 7.4
Volume-900ml, Sample volume-2ml
Temperature - 37±0.5 °C
RPM-50
SGF pH 1.2 was transferred in dissolution vessels and the temperature and rpm were set at 37±0.5 °C and 50 respectively. The developed tablets were transferred to dissolution vessels. A 2ml of aliquots of dissolution fluid were withdrawn after 2h and the medium was replaced with phosphate buffer pH 6.8.
The samples were withdrawn for 3h and again the medium was replaced with phosphate buffer pH 7.4. Samples were withdrawn for 24h, suitably diluted and analyzed using double beam UV-Visible spectrophotometer (Shimadzu-160A) at 331nm.
Evaluation of core tablets: Core tablets were evaluated for various parameters which are shown in Table 3 & 4.


Evaluation of coated tablets: Coated tablets were evaluated for general appearance and the results are shown in Table 5.

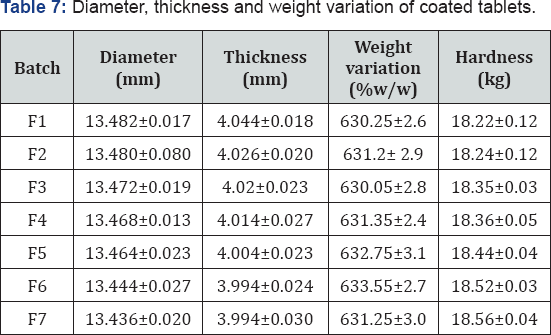
Various physical parameters of prepared tablets were shown in Table 6. Diameter and thickness of all the formulated batches ranged from 13.436 to 13.482mm and 3.99 to 4.04mm respectively. All the formulated batches pass the weight variation test. Hardness of all the formulated batches ranged from 18.22 to 18.56kg Table 5 showed that as the concentration of gum increases hardness of tablet increases.

Drug release profile of all the formulated batches was determined via USP Dissolution Apparatus II paddle type and shown in Figure 1.
The results indicated that as the almond gum concentration was increased release of drug from formulation decreased up to F5 batch and then drug release was increased up to certain level. No drug was released in the acidic medium; at pH 1.2. All formulations showed a very slow release of the drug depending on the Almond gum composition. After 8 h less than 30% of drug was released from tablets containing 90-240mg of Almond gum (F1-F6). Formulation containing 270mg of Almond gum (F7) exhibited the highest drug release rate then the F1 batch. F4 batch was selected as the best batch because it released the 97% of drug over the period of 24h. This dissertation work proved that 5-ASA compression-coated with 30% Almond gum was found to be a promising drug delivery system for treatment of inflammatory bowel disease (Table 7).
Conclusion
Seven batches (F1-F7) were prepared by varying the concentration of almond gum. The compression coated tablet was coated with ethyl cellulose (4%) as delayed release coating. The developed formulation was evaluated for various tests like diameter, thickness, hardness, friability, weight variation, drug content and In vitro drug release. In vitro drug release was carried out at three different pH conditions (1.2, 6.8 and 7.4) simulating the condition of GIT. F4 batch was selected as the best batch because it releases the 97% of drug over the period of 24h. A 4% ethyl cellulose coating was sufficient to delay the release for 5h. it can be concluded from the complete study that Prunus amygdalus gum can be effectively used as compression coated material for the colon targeting of drug.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment