Drug Delivery Development: Quality Concepts, Challenges and Prospects-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
Quality, being a key to success in competitive market is an imperative indicator of product. It is important to recognize that quality cannot be tested into products, i.e., quality should be built in by design. The search for new drug delivery approaches and new modes of action is a rapidly developing field. Modes of drug delivery have changed in the past few decades and the future looks set to provide even more therapeutic advances. DDS can be developed to target common and rare diseases; both come with their challenges and opportunities, which are explored in this article.
Challenges in quality drug development mainly includes negligence from management, material management, quality personnel, lack of validated process, lack of equipment qualification, improper documentation, research validation specially related to novel drug delivery system. Nevertheless these challenges can be reduced to an appreciable level with the proper training, initiatives from management, continuous validation programmes and accepting novel drug delivery development considering risk assessment.
Keywords: NDDD; Quality issues in NDDD; Drug development challenges
Introduction
The quality in the pharmaceutical industry has become a major concern and there has been a growing awareness for the significance of the quality of the pharmaceutical products. The current concept of Good Manufacturing Practices (GMP) accentuates that the quality of pharmaceutical products must be constructed during the overall process cycle [1]. Quality is never improved in a common way. It is always improved project by project, beginning with the most significant problems [2]. The deficiency to be challenged must be clearly specified & the expected improvement can be defined in measurable terms [3]. This article examines the current status of quality related issues in development of drug delivery system with the objectives of assessing challenges and prospects.
Challenges
Challenging molecules and challenging markets is the key factor in drug development process. "On the molecule side, the pipeline is full of molecules with bioavailability, stability, targeted delivery, controlled release, and manufacturability challenges [4,5]. The benefit to risk ratio seems far removed and in true sense there is a functional gap between the development function and technical operations in the drug companies [6]. Cost of quality involves prevention cost, appraisal cost, internal failure cost (Scrap, rework and material losses) and external failure cost (returns and recalls), which has been neglected in the development process of delivery systems [7]. Global regulatory trends are yet to be defined fully, despite the several attempts already performed specially for novel drug delivery systems like Nano medicines. The other crucial issue is scale up. Commercial manufacturing uses much larger quantities compared to supply for the laboratory-scale experiments and, therefore, is a different ball game. Raw material batch-to-batch variability needs to be understood, process capabilities evaluated, and controls demonstrated by the vendor [8-10].
Prospects
The physicochemical and biological properties of the drug substance that can influence the performance of the drug product and its manufacturability should be identified and discussed [11]. The information on excipient performance can be used, as appropriate, to justify the choice and quality attributes of the excipient and to support the justification of the drug product specification [12]. Supportive management (philosophically and financially) can bring a quality concept and develop quality culture in the employees [13]. Quality policies need to be adopted indicating the goals of organization and support system in place to achieve those goals. Responsive deviation and investigation systems that lead to timely remediation will reduce the batch to batch variations [14].
Well-defined, designed and validated processes during entire product development life cycle can assure the product quality and reproducibility. While the rules and guidelines are quite well in place, there exists a non-uniform interpretation of these rules. An emphasis on the training of continuous manufacturing technologies, regulatory and organizational approaches is the need of hour in development of novel drug delivery system [13]. Fostering voluntary compliance by the researcher should be the focus rather than adopting more strict regulations [14]. A full scale design of experiment (Quality by Design) that begins with predefined objectives should be considered in the developmental strategy. It highlights product and process understanding and process control, based on Science-based approaches and sound methods for assessing risk [8]. This systematic approach can enhance achieving the desired quality of the product and helps in understanding manufacturing strategy. Process development studies should provide the basis for process improvement, process validation, continuous process verification (where applicable), and any process control requirements as given in Figure 1 [15].
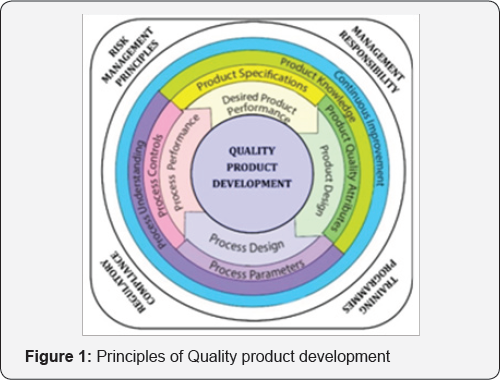
Discussion
A systematic process plan and plant design undertaken by management and its implementation by considering trainings and research validation as part of the program can definitely lead to a quality product. QbD is just an approach to design a robust product but a quality product can be achieved only with the agreement of ethical principles for voluntary compliance rather than just fulfilling regulatory and organizational goals.
Conclusion
Quality drug development is not a rocket science but it's just willingness of researchers involving certain principles to be followed.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment