Multidrug Liposomes of Glycolic Acid and Nutraceuticals for Cosmetic Application-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
Glycolic acid is used to improve skin aging and in combination with nutraceuticals like vitamins C or E, it may provide synergestic effect for skin protection. Liposome formulation were prepared by film hydration method and studied for different test parameters like zeta potential, particle size, entrapment efficiency and in vitro release studies. The particle size and zeta potential were found to be in range of 200-900 nm and ±14mv to ±29mv. The entrapment efficiency was found to be between 16-76% and in vitro drug release was in controlled manner. Liposome formulation containing vitamins E and C, and cholesterol was found to be most optimum and effective. Hence, Liposomes composed of vitamin E and vitamin C can be used as an alternative to cosmetic applications.
Keywords: Liposomes; Glycolic acid; Vitamin C; Vitamin E; Cosmetics
Introduction
Many significant studies have been conducted and applied to use the potential lipid-based drug delivery system, as it provides a suitable means for controlled release. Lipid-based carriers are efficient and safe hence they can be an attractive candidate for formulation of pharmaceuticals and nutraceutcials [1]. Liposomes are microscopic bilayered and spherical-shaped vesicles containing an internal aqueous compartment [2]. Presently cosmetic products mostly contain liposomes as it can encapsulate active ingredients to store and prolong release which is necessary for the skin. The cosmetics of liposomes are applied to the skin; it is deposited on the skin and activated to fuse with the cellular membranes. Such formulations with unsaturated phosphatidylcholine are selected for skin regeneration, antiaging, anti-acne, and skin penetrant like vitamins and their derivatives into the skin [3].
The combinational treatment of pharmaceuticals with nutraceuticals provides accelerated therapeutic action enhanced bioavailability and improved biological application of the cosmetics. Nutraceutical-based topical delivery systems can be formulated as a functional cosmetic to complement efficacy of the formulation [4]. Glycolic acid is generally used as exfoliant, moisturizer and efficacious in increasing skin elasticity; this action is probably due to the direct stimulation in production of collagen, elastin and mucopolysaccharides in the deeper layers of the skin [5]. Glycolic acid is known to dissolve adhesion between cells in the upper layers of the skin, including shedding of dry scales from the skin's surface, which is referred as exfoliating effect [6-8].
Skin is protected from the harmful effects of free radicals due to the presence of antioxidants consisting of variety of lipophilic (Vitamin E) and hydrophilic (Vitamin C) and it is responsible for balancing pro-oxidants and antioxidants [9]. Antioxidants like ascorbic acid are playing an important role in neutralizing reactive oxygen species that includes singlet oxygen, hydroxyl radical, and superoxide anion. After neutralization, they form ascorbic acid free radical which shows comparatively less pro-oxidant activity. This ascorbic acid free radical further gets converted to L- ascorbic acid. Vitamin E is responsible for protection of skin against UVA as well as UVB radiations. It helps to protect skin against oxidative stress damage that is caused upon solar exposure. Vitamin E, Vitamin C and their combination have shown positive results as topical photo-protectant [10-12].
Use of liposomes in topical drug delivery includes many benefits like reduction of side effects, incorporate both hydrophilic and hydrophobic drugs, controlled release and improves potential for topical application [13,14]. Liposomes are also used in cosmetics for skin care preparations applied in form of gels of solutions. [15] So, the objective of this research work was to formulate and characterize multidrug liposomes of glycolic acid and nutraceutical for cosmetic application. Cholesterol was added to different combination of liposomes to enhance the stability and hardness of the formulation.
Methods and Materials
Materials
All the chemicals used in the experiment were purchased from Glycolic acid was purchased from S.D. fine chem limited, Mumbai, India. Cholesterol was purchased from Central Drug House (p) ltd, New Delhi, India. Soya lecithin was from HiMedia Laboratories Pvt Ltd, Mumbai, India.Vitamin C was from Molychem, India. Vitamin E was from Merck Specialities Pvt Ltd, Mumbai, India. Chloroform was purchase from Researchlab, Mumbai, India.
Method
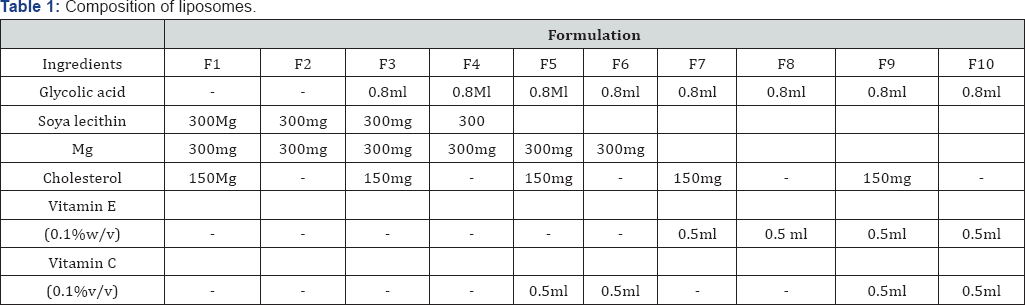
The liposomes were prepared by film hydration method. Accurately weighed soya lecithin and cholesterol were weighed and placed in around bottom flask. Chloroform was added to it in order to dissolve the present contents. Glycolic acid was then added to the solution and 0.5ml of 0.1% w/v solution of vitamin C and vitamin E was added to the formulation. The flask was attached to rotary evaporator until a thin film of liquid was formed. Distilled water was added to it in the portion of 20, 30, 50ml at the interval of 10mins. Mixing was continued on rotary evaporator. Contents were stirred using magnetic stirrer, if required. Liposomes were formed with and without cholesterol along the combination of vitamins. Formulations F2, F4, F6, F8 and F10 are liposomes without cholesterol whereas formulations F1, F3, F5, F7 and F9 contain cholesterol. Formulation F5 and F6 contains vitamin C, F7 and F8 contains vitamin E and F9 and F10 contains both the vitamins. The composition of the formulation is shown in Table 1.
Characterization of liposomes
Particle size and zeta potential
The particle size and zeta potential of liposomes were determined using zeta size (Malvern Zetasizer, UK). The samples were dispersed in water and measured at 25 °C.
Capsulation efficiency
Ten ml sample was centrifuged at 8000 rpm for 10 mins. Accurately measured 100μl of supernatant was diluted with 5ml of distilled water and absorbance was measured. The precipitate was sonicated. 100μl was diluted with 5ml of distilled water and absorbance was measured. The entrapment efficiency was measured by the following formula; % capsulation efficiency= [concentration of precipitate / (concentration of supernatant+ concentration of precipitate)] x 100
In vitro drug release
One ml of liposomes was packed in cellophane membrane (Himedia, India) with pore size 0.45nm which was placed in 30 ml of phosphate buffer pH 7.4. Five ml of aliquots was withdrawn at pre-planned intervalsof 1h, 2h, 4h, 8h, and 24h. Absorbance was measured using UV-Vis spectraphotometer and drug release was calculated at 226nm.
Result
The present studies were carried out to develop liposomes of 10 different formulations (F1, F2, F3, F4, F5, F6, F7, F8, F9, F10) containing different nutraceuticals by using film hydration method.
Particle size and zeta potential
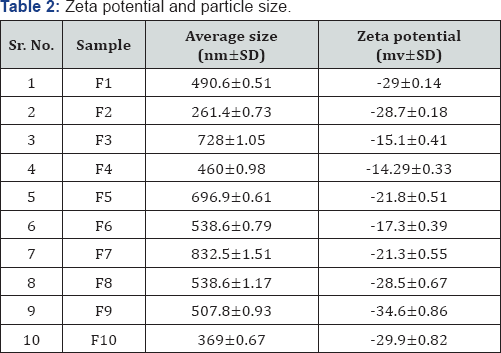
The zeta potential and particle size of liposomes was measured using (Malvern Zetasizer, UK) as shown in Table 2.
Entrapment efficiency
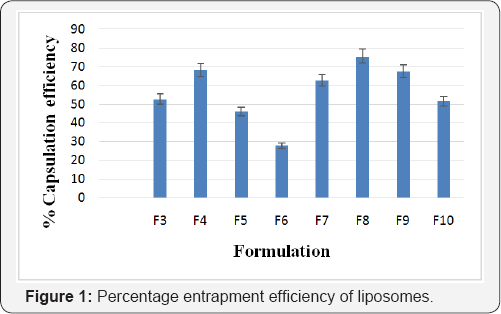
The % entrapment efficiency of glycolic acid liposomes of formulations F1 to F10 was found to be in the range 27.8±2.34 to 75.5±3.17%. The entrapment efficiency of formulations was in order of F6<F5<F10<F3<F7<F9<F4<F8 as shown in Figure 1.
In vitrodrug release study
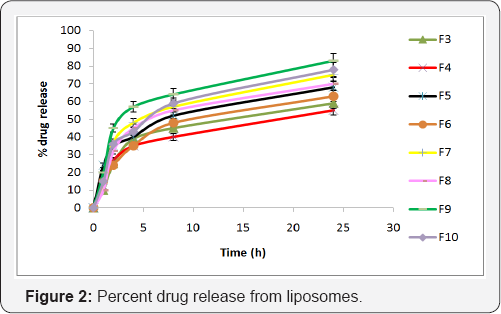
In vitro release studies were performed using dialysis membrane method using phosphate buffer pH 7.4. The % release of glycolic acid from formulations F1 to F10 was measured at 226nm as shown in Figure 2.
Discussion
Liposome formulation containing glycolic acid with different nutraceuticals (vitamins C and E) were prepared and evaluated for particle size, zeta potential, entrapment efficiency and %drug release. Formulations F1 and F2 were considered as placebo as they were without glycolic acid. Formulation F3 showed highest particle size of 728±1.05nm and formulation F10 showed lowest particle size of 369±0.67nm. Formulation F9 showed the lowest zeta potential of -34.6±0.86 and formulation F4 showed the highest zeta potential of -14.29±0.33. Formulations containing cholesterol have larger particle size and lower zeta potential than formulations without cholesterol. The entrapment efficiency results showed that majority of liposomes have appreciable entrapment efficiency. Formulation F8 was observed to possess the highest entrapment efficiency with 75.5±3.17%.The entrapment efficiency of formulations was in order of F6<F5<F10<F3<F7<F9<F4<F8. In vitro drug release of all the formulations was carried out and it was observed that formulation F9 showed 83.2±2.35% of drug release from the liposomes by 24 hrs. Formulations containing cholesterol were found to have lower entrapment efficiency and drug release than the ones without cholesterol.
Formulation F9 containing vitamin E, vitamin C and cholesterol was found to be most optimum and effective when compared to other formulations. Hence, by changing the ratio of soya lecithin and cholesterol, size of liposome can be attained in nanoscale and possess improved drug entrapment efficiency and controlled drug release.The prepared liposomes can be added to gel or transdermal patch to obtain topical drug delivery. Glycolic acid provides excellent exfolient effect and moisturizing effect. It provides possibilities for using glycolic acid in cosmetic application. Moreover, the combination of pharmaceutical and nutraceuticals further enhances the biological action, bioavailability and biological application of cosmetics.
Conclusion
Liposomes can be used in dermal applications in form of protective systems for active ingredients. They also have moisturizing properties. Multidrug liposomes of glycolic acid and nutraceutical for cosmetic application were successfully prepared and evaluated. Liposome formulation containing vitamins E and C, and cholesterol was found to be most optimum and effective. Hence, Liposomes composed of vitamin E and vitamin C can be used as an alternative to cosmetic applications.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment