Functional Selective D2 Ligands for the Treatment of Schizophrenia-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
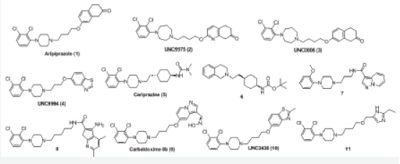
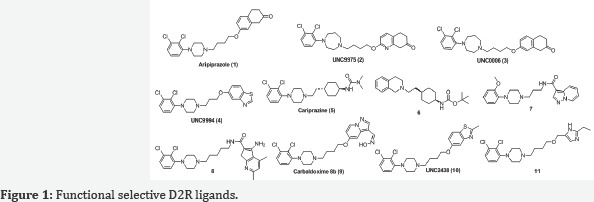
Functionally selective ligands (also known as biased ligands) of dopamine D2 receptors have been considered as not only valuable tools for dissecting the roles of D2-mediated G protein-dependent and independent signaling pathways, but also better antipsychotic drug candidates for neurological and psychiatric disorders including schizophrenia. Consequently, functionally selective D2R ligands have also been increasingly pursued by the biomedical community as promising antipsychotic therapeutics with improved efficacy and reduced side effects compared with unbiased ligands. This review will discuss the recent development in the discovery of functional selective D2R ligands Figure 1.
Introduction
Schizophrenia is a chronic and severe mental disorder characterized by abnormal social behavior and failure to understand reality [1]. Clinically, the disorder manifests with a large variety of symptoms that fall into three categories: positive, negative, and cognitive [2]. Schizophrenia affects about 1.1% of world wide population [3]. Although schizophrenia is not as common as other mental disorders such as anxiety disorder (18.1%) [4], depression (6.9%) [5], and bipolar disorder (2.6%) [6], the symptoms can be very disabling. Therefore, it is often associated with high levels of morbidity and mortality [7]. The average life expectancy of people with schizophrenia is ten to twenty-five years less than for the general population [8]. This is the result of increased physical health problems and a higher suicide rate (about 10%) [9,10].
Although scientists believe that a combination of genetics, environment, and altered brain chemistry and structure may play a role in the development of schizophrenia [11], the exact cause of this disorder is still unknown to the research community. Consequently, there is no cure for this disorder and treatments have been focusing on eliminating the symptoms of the disease. The primary treatment of schizophrenia is antipsychotic medications [12], often in combination with psychological and social supports. While the current FDA-approved antipsychotic drugs are able to reduce the positive symptoms of schizophrenia in about 7 to 14 days [13,14], they have limited efficacy with negative and/or cognitive symptoms [15,16].
Furthermore, antipsychotic medications can greatly lower the patients' life quality by inducing a wide range of motor, metabolic, cardiovascular, and emotional side effects which also lead to treatment noncompliance [14,17]. Therefore, it is of great necessity to develop novel pharmaco therapies which have better efficacies for treating all three clinical schizophrenia symptoms and possess a low profile for side effects.
With all currently FDA-approved antipsychotic drugs target primarily the dopamine D2 receptor (D2R) for their reaction [15-18], it is indicated that opposing dopamine signaling is central for alleviating psychotic symptoms with schizophrenia [19]. Clinical observations have revealed that schizophrenia-like symptoms occur in amphetamine abusers due to excessive dopamine release [20]. Also baseline dopamine levels and stimulated release of dopamine are found to be abnormal in mesolimbic systems of brains from schizophrenic patients [21]. All these evidences have put dopamine receptor, especially dopamine D2R at the center for the development of antipsychotic drug. Antagonists and partial agonists of D2R have been extensively pursued as antipsychotic therapeutics for the treatment of schizophrenia [22,23].
As a member in the big family of G protein-coupled receptors (GPCR), dopamine D2 receptor (D2R) is Gαi/o coupled whose activation inhibits CAMP production [24]. Antipsychotic drugs targeting D2R were originally identified as being able to bind to D2R and regulate CAMP synthesis [18]. Mounting evident from recent studies indicate that D2R signal not only via canonical pathway involving hetero trimeric large G protein, but also via noncanonical G protein-independent pathways with other signaling proteins including, most prominently, β -arrestins [2528]. Most antipsychotic drugs have been found to have both G protein-dependant and G protein-independent actions [28]. The process by which GPCR ligands, including D2R ligands, differentially modulate canonical and noncanonical signal transduction pathways is a phenomenon known as "functional selectivity" or "biased agonism" [29,30].
With functionally selective D2R ligands that can preferentially activate either canonical or noncanonical D2 signaling pathways [31,32], it is thus very clear that the D2R ligands with discrete functional selectivity profiles will be extremely useful for elucidating the key signal transduction pathways essential for both the therapeutic actions and the side effects of antipsychotic drugs [30]. And this understanding will in turn enable the design of better antipsychotic drug candidates, which can ultimately lead to safer and more effective therapies for schizophrenia patients. In this short review article, we will summarize the recent development in the discovery of functional selective D2R ligands for the treatment of schizophrenia.
Functional Selective D2RLigands
Aripiprizole
Aripiprazole (OPC-14597, 1), an FDA-approved atypical antipsychotic drug, was one of the first functionally selective D2R ligands identified [31,33,34]. It has an excellent side-effect profile presumed, in part, to be due to lack of typical D2R antagonist properties. Although aripiprazole was initially described as a partial D2R agonist, on the basis of assays performed in whole animals and isolated tissues [35-37], it was later demonstrated that aripiprazole could behave as a full agonist, a partial agonist, or an antagonist at D2R depending upon the signaling readout and cell type interrogated [28,31,33,38].
The study examined D2R binding properties of aripiprazole as well as the effects of the drug on three downstream D2R- mediated functional effectors including mitogen-activated protein kinase (MAPK) phosphorylation, potentiation of arachidonic acid (AA) release, and D2R internalization revealed that aripiprazole affects D2L-mediated signaling pathways in a differential manner [31]. In the study examining the properties of aripiprazole at D2 like auto receptors by monitoring the changes of dopamine synthesis in adult rat brain striatal minces incubated ex vivo, it was found that alteration of dopaminergic tone by depolarization affected the actions of aripiprazole on D2- like auto receptors [39].
The in vivo study aimed to investigate the effects of aripiprazole on the D2R downstream cAMP-PKA and Akt-GSK3 β signaling pathways suggested that aripiprazole had differential effects on the cAMP-PKA and Akt-GSK3 β signaling pathways in the brain areas [40]. Additional studies examined the activity of aripiprazole in D2R-mediated heterologous sensitization of adenylyl cyclase and cell-based dynamic mass redistribution (DMR). Aripiprazole displayed a unique functional profile for modulation of G proteins, being a partial agonist for Gαi/o and a robust antagonist for G β γ signaling. Additionally, aripiprazole was a weak partial agonist for both heterologous sensitization and dynamic mass redistribution [41].
UNC9975 (2), UNC0006 (3) and UNC9994 (4)
Through a robust diversity-oriented multi-dimensional modification ofthe scaffold represented by aripiprazole (1), Three β -arrestin-biased D2R ligands including UNC9975 (2), UNC0006 (3), and UNC9994 (4) were reported as the unprecedented β -arrestin-biased ligands for a Gi-coupled G protein-coupled receptor (GPCR) [42]. All three D2R ligands were simultaneously antagonists of Gi-regulated CAMP production and partial agonists for D2R/β-arrestin-2 interactions. Importantly, UNC9975 displayed potent antipsychotic-like activity without inducing motoric side effects in inbred C57BL/6 mice in vivo. Genetic deletion of β -arrestin-2 simultaneously attenuated the antipsychotic actions of UNC9975 and transformed it into a typical antipsychotic drug with a high propensity to induce catalepsy.
Similarly, the antipsychotic-like activity displayed by UNC9994, an extremely β -arrestin-biased D2R agonist, in wild- type mice was completely abolished in β -arrestin-2 knockout mice. These results suggest that β -arrestin signaling and recruitment can be simultaneously a significant contributor to antipsychotic efficacy and protective against motoric side effects [42]. Follow-up comprehensive structure-functional selectivity relationship studies (SFSR) focused on four regions of aripiprazole scaffold (e.g. left hand side phenyl region, middle amino region, central linker region, and right hand side bicyclic aromatic region) resulted in more β -arrestin biased D2R agonists [43].
This combined medicinal chemistry and pharmacological profiling approach also provided the biomedical community a successful proof-of-concept for how functionally selective ligands can be discovered. The study designed to test the effectiveness of UNC9975 or UNC9994 on schizophrenia-like behaviors in phencyclidine treated or NR1-knockdown hypoglutamatergic mice showed that the UNC compounds reduced hyper locomotion in the open field, restored PPI, improved novel object recognition memory, partially normalized social behavior, decreased conditioned avoidance responding, and elicit a much lower level of catalepsy than haloperidol [44]. These preclinical results suggest that exploitation of functional selectivity may provide unique opportunities to develop drugs with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating schizophrenia and related conditions than medications that are currently available.
Cariprizine (5) and analogs (Compounds 6 And 7)
Cariprizine(5) is an atypical antipsychotic recently approve by FDA for the treatment of schizophrenia and bipolar mania [45]. It acts primarily as a partial agonist for D2R and D3R [46] with higher selectivity for D3R [46,47]. The structure-functional selectivity relationship (SFSR) studies of cariprazine scaffold carried out by Shonberg and colleagues at Monash University was focused on three main portions of the lead compound: the tertiary amine containing "head group", the cyclohexylene "spacer" group, and the tert-butyl carbamate "tail group" [48].
Similar to the SFSR studies of aripiprazole analogs, to assess G protein-related signaling, the compounds were profiled in the D2 CAMP accumulation assay, whereas β -arrestin signaling was evaluated by measuring phosphorylation of extracellular signal-regulated kinase 1/2 (ERK 1/2). In this testing paradigm, cariprazine (5) displayed a 230-fold bias toward the cAMP pathway compared with dopamine. Interestingly, while all cariprazine derivatives disclosed in this letter showed a bias toward the G protein signaling pathway (6), subtle changes of the D2 unbiased partial agonist, a structurally related aryl piperazine reported by Tschammer [49], led to analogues that displayed a strong bias toward β -arrestin signaling such as compound 7. By combining the medicinal chemistry efforts with novel analytical pharmacology methods [50], it was discovered that the nature of the head group, the composition of the tail group, and the orientation, length, and flexibility of the spacer were all important factors for the control of functional selectivity at the D2R [48].
Functional selective D2R ligands with privileged structures (compound 8)
Another comprehensive structure activity relationship (SAR) studies carried out by Szabo and colleagues at Monash University also led to the discovery of novel functional selective D2R ligands [51]. They investigated the determinants of efficacy, affinity, and bias for three privileged structures for the D2R, exploring changes to linker length and incorporation of a heterocyclic unit. After profiling the newly synthesized compounds in two signaling assays (CAMP and pERK1/2), they were able to identify and quantify determinants of functional selectivity at the D2R. The results from combined medicinal chemistry and pharmacological profiling approach revealed that: 1) substitution on the phenylpiperazine privileged structures (2-methoxy vs 2,3-dichloro) influenced bias when the thienopyridine heterocycle was absent; and 2) upon inclusion of the thienopyridine unit, the substitution pattern (4,6-dimethyl vs 5-chloro-6-methoxy-4-methyl) had a significant effect on bias that overruled the effect of the phenylpiperazine substitution pattern [51]. This latter observation could be reconciled with an extended binding mode for these compounds, whereby the interaction of the heterocycle with a secondary binding pocket may bring functional selectivity to the parent compounds. The resulted novel D2R partial agonists, 8 and 9, display a similar affinity for the D2R as aripiprazole but exhibit distinct functional selectivity profiles, which made them useful tools to explore the contribution of functional selectivity to antipsychotic efficacy.
Functional selective D2R/D3R partial agonists
Dopamine D2R and D3R are known as valuable targets for the treatment of neurological and psychiatric disorders including schizophrenia [52-54]. Hiller and colleagues evaluated a series of newly synthesized compounds for their ability to differentially activate distinct signaling pathways [55].
Measurement of D2L- and D2S-mediated [35S] GTPyS incorporation in the presence of coexpressed Gao and Gai subunits showed significantly biased receptor activation for several test compounds [55]. A follow-up study from the same group using the same evaluation methods yielded the most striking functionally selective D2R ligand, carbaldoxime 8b (9, Gaol, pEC50 = 8.87, Emax = 65%; Gai2, pEC50 = 6.63, Emax=27%) [56]. It was also indicated in the study that 1,4-disubstituted aromatic piperazines (1,4-DAPs) behaved as antagonists for β -arrestin-2 recruitment, implying significant ligand bias for G-protein activation over β -arrestin-2 recruitment at D2R. Regiochemistry and the nature of functional groups attached to the pyrazolo [1,5-a]pyridine moiety strongly influence ligand efficacy and selectivity between D2R and D3R activation [56].
Other functional selective D2 ligands
Intensive structural exploration on aripiprazole scaffold engendered UNC2438 (10) which was found to be a G-protein biased compounds [57]. Further structure-functional selectivity relationship studies (SFSR) on UNC2438 focused on the right hand side benzothiazole moiety and the left hand side phenylpiperazine moiety furnished a few additional G-protein biased compounds which were partial agonists in D2R Gio- mediated CAMP inhibition assay and simultaneously inactive in D2R-mediated β -arrestin-2 recruitment assay [57].
Most recently, a tailored virtual library with close to 13,000 compounds bearing 2,3-dichlorophenylpiperazine, a privileged orthosteric scaffold, connected to diverse chemical moieties via a linker was docked to the D2R model [58]. Eighteen top-ranked compounds that occupied both the orthosteric and allosteric site were synthesized, leading to the discovery of 16 partial agonists. While a majority of the ligands had comparable maximum effects in the G protein and β -arrestin recruitment assays, some displayed preference for a single pathway. In particular, compound 11 stimulated β -arrestin recruitment (EC50 = 320 nM, Emax = 16%) but had no detectable G protein signaling.
Conclusion
The growing realization of the complexity of G protein coupled receptor (GPCR) mediated signal transduction pathways, specifically D2R mediated signaling pathways, has provided a theoretical framework for the development of functionally selective or biased ligands. Several studies have demonstrated that agonists differ in their ability to activate various pathways. Notably, blockade of β arrest in recruitment was found to be a shared property of antipsychotics that exhibit either antagonist (e.g., haloperidol), or partial agonist (e.g., aripiprazole) activity through Gai/o-CAMP pathways [28]. In contrast, a study with analogs of the novel antipsychotic aripiprazole suggested that D2 ligands with Gai/o antagonist and β -arrestin agonist activity may have antipsychotic behavioral activity with reduced extra pyramidal side effects in a mouse model [3]. While there are still a lot of questions to be answered in this field, heightened awareness of the potential benefit of pathway biased D2R ligands has inspired scientists in this area develop more functionally selective D2R ligands as better antipsychotic therapies for schizophrenia.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/


Comments
Post a Comment