PPARα as Potential Therapeutic Target for Neurodegenerative Diseases-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DRUG DESIGNING & DEVELOPMENT
Abstract
Peroxisome proliferator activated receptor a (PPARα) is ligand-activated transcriptional factor receptor belonging to nuclear receptors family. It plays a key role in lipid metabolism and glucose homeostasis and it is important in the prevention and treatment of metabolic diseases. PPARα has also a protective effects against brain cell death attributed to its anti-inflammatory and antioxidant properties. In the present work, we discuss the PPAR involvement in neurodegenerative pathologies and its potential as therapeutic target for these diseases.
Keywords: Alzheimers disease; Parkinsons disease; Neuroprotection
Abbreviations: PPARs: Peroxisome Proliferator Activated Receptors; RXR: Retinoid X-Receptor; SARs: Structure Activity Relationships; PEA: Palmitoyl Ethanol Amide; LPS: Lipo Poly Saccharide.
Mini Review
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptors super family and are ligand-activated transcription factors. They are involved in the regulation of metabolic pathologies such as cardiovascular disease, obesity, lipid disorder, hypertension and diabetes [1]. PPARs exist as three subtypes commonly designated as PPARα, PPARγ, and PPARβ/δ. All PPAR isoforms, once within the nucleus, heterodimerize with retinoid X-receptor (RXR) and bind to specific DNA-response elements in the promoter of target genes. When a ligand binds to PPARs, there is a conformational change in the receptor that causes the removal of co-repressors and the recruitment of co-activators; this causes chromatin remodeling which allows the initiation of DNA transcription [2].
PPARα, PPARγ, and PPARβ/δ are expressed in different tissues and with distinct binding ligands, co-activators or corepressors. PPARα, mainly expressed in tissues involved in lipid oxidation such as kidney, liver, skeletal and cardiac muscle, plays an important role in fatty acid oxidation and lipoprotein metabolism; PPARγ is expressed predominantly in adipose tissue and vascular smooth muscles; PPAR β /δ is expressed broadly and particularly in tissues associate with fatty acid metabolism, but also in the small intestine, liver, colon and keratinocytes [3]. A lot of studies showed that PPARs are expressed also in brain and in particular in neurons and glia [4]; for this reason, the potential use of PPAR agonists as neuroprotective agents in neurodegenerative disorders has been suggested. Neurodegenerative diseases are incurable pathologies with a progressive degeneration of neurons associated with motor and cognitive damage. These conditions are characterized by oxidative stress, mitochondrial and transcriptional dysregulation and apoptosis [5]. Because oxidative stress and neuro inflammation are involved in cell death, these dysfunctions are the key factors for the development of the most common neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis.
Therefore, novel therapeutic approaches are useful to obtain a reduction of the symptoms and slow progression of the pathology. In this contest, the role of PPARα is emerging as a promising pharmacological target for the treatment of neurodegenerative diseases. Fibrates are PPARα agonists widely studied especially for the treatment of hyperlipidemias (Figure 1). Some of these, such as gemfibrozil, ciprofibrate, WY-14643 or fenofibrate, activate selectively only PPARα, others do not have an isoform selectivity. For example, GFT505 is a dual PPARα/δ agonist and bezafibrate, that actives all three isoforms, is a PANagonist [6].
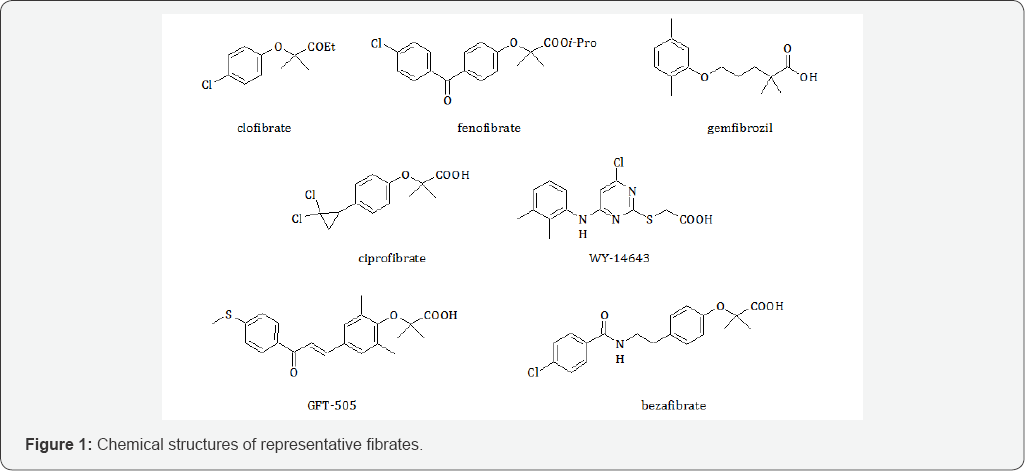
In the last years, the development of new fibrates that activate PPAR has been an important objective to better understand structure activity relationships (SARs) for obtaining new drugs with a better pharmacological profile [7,8]. In this contest, the neuroprotective effects of PPARα agonists have been studied; some researchers attributed this effect largely to the PPARα antioxidant and anti-inflammatory properties but also to the positive effects in lipid metabolism and glucose homeostasis [9].
About anti-inflammatory properties of PPARα, it was showed especially in astrocytes and microglia [10,11]. In fact, several authors demonstrated that the use of PPARα agonists, such as ciprofibrate, fenofibrate, gemfibrozil and WY-14643, causes a reduction of NO production especially in mouse microglia stimulated by lipopolysaccharide (LPS). Furthermore, it has been demonstrated that the treatment with palmitoylethanolamide (PEA) causes a reduction of oxidative stress in astrocytes mediated by PPARα [12]. PPARα is also expressed in brain and the anti-inflammatory role was evidenced by reduction of LPS- induced TNFα , IL-1β, IL-6 and COX-2 [13].
The anti-inflammatory effect mediated by PPARα has also been identified in reactive astrocytes. It has been shown that PPARα attenuates the inflammation in reactive astrocytes by decreasing NO and pro-inflammatory cytokines. Additional, PPARα has an important role in other glial cells such as microglia and ependymal cells in response to injury. [14]. PPARα has an antioxidant effect associated with a reduction of cerebral oxidative stress depending on the increase in activity antioxidant enzymes, such as Cu/Zn superoxide dismutase and glutathione peroxidase. This activity causes a decrease in lipid peroxidation and ischemia-induced reactive oxygen species production [9].
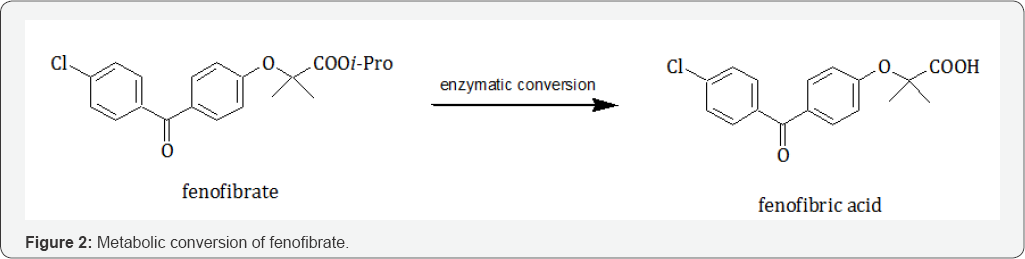
The anti-inflammatory and antioxidant properties of PPARα explain the neuroprotective effects especially in Parkinson's disease and Alzheimer's disease [15]. For these reasons, PPARα could be a therapeutic target for Parkinson’s disease; in fact, it has been established that there is a neuroprotective effect in the brain of animals treated with fenofibrate by decreasing inflammation. Uppalapati et al. showed that fenofibric acid, the active metabolite of fenofibrate (Figure 2), was present in the brain of animals treated with fenofibrate, suggesting that this compound was metabolized and that crossed the blood-brain barrier in vivo [16].
It was discovered that fenofibrate prevent the dopaminergic neurons loss in the substantia nigra, and it attenuates the loss of tyrosine hydroxylase immune reactivity in the striatum [17]. Many studies have shown that PPARα could have a therapeutic effect also in Alzheimer's disease, even if this conclusion remains controversial. Some researchers demonstrated that PPARα has a protective effect against beta-amyloid-induced neurodegeneration [18], but others found that fenofibrate increases beta-amyloid production in vitro; perhaps this effect of fenofibrate is not connected with PPARα activation [19]. Further, PPARα activation induces vascular protection through an improvement of cerebral artery sensitivity [20].
To conclude, neurodegenerative diseases induce progressive loss of cognitive functions and current drugs only furnish temporary symptomatic alleviation without blocking disease progression. During last years, growing interest was directed towards PPARs that have the capability to positively regulate the genes expression with the aim to modulate several molecular pathways responsible of neurodegenerative diseases. In particular, different researchers have shown the positive involvement of PPARα in neurodegenerative disease.
The useful effects are principally due to PPARα antiinflammatory and antioxidant properties but also to the capacity to restore the vascular and endothelial integrity. The potential use of PPARα agonists as neuroprotective agents against neurodegenerative disorders is an important start point to find new drugs that could cure definitively these pathologies. Though more laboratory and clinical studies are needed to understand all mechanisms involved in the neuroprotective actions of the PPARα agonists, these receptor is an effective target for neurodegenerative disorders.
For more Open Access Journals in Juniper Publishers please click on: https://www.crunchbase.com/organization/juniper-publishers
For more articles in Open Access Novel Approaches in Drug Designing & Development please click on: https://juniperpublishers.com/napdd/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/



Comments
Post a Comment